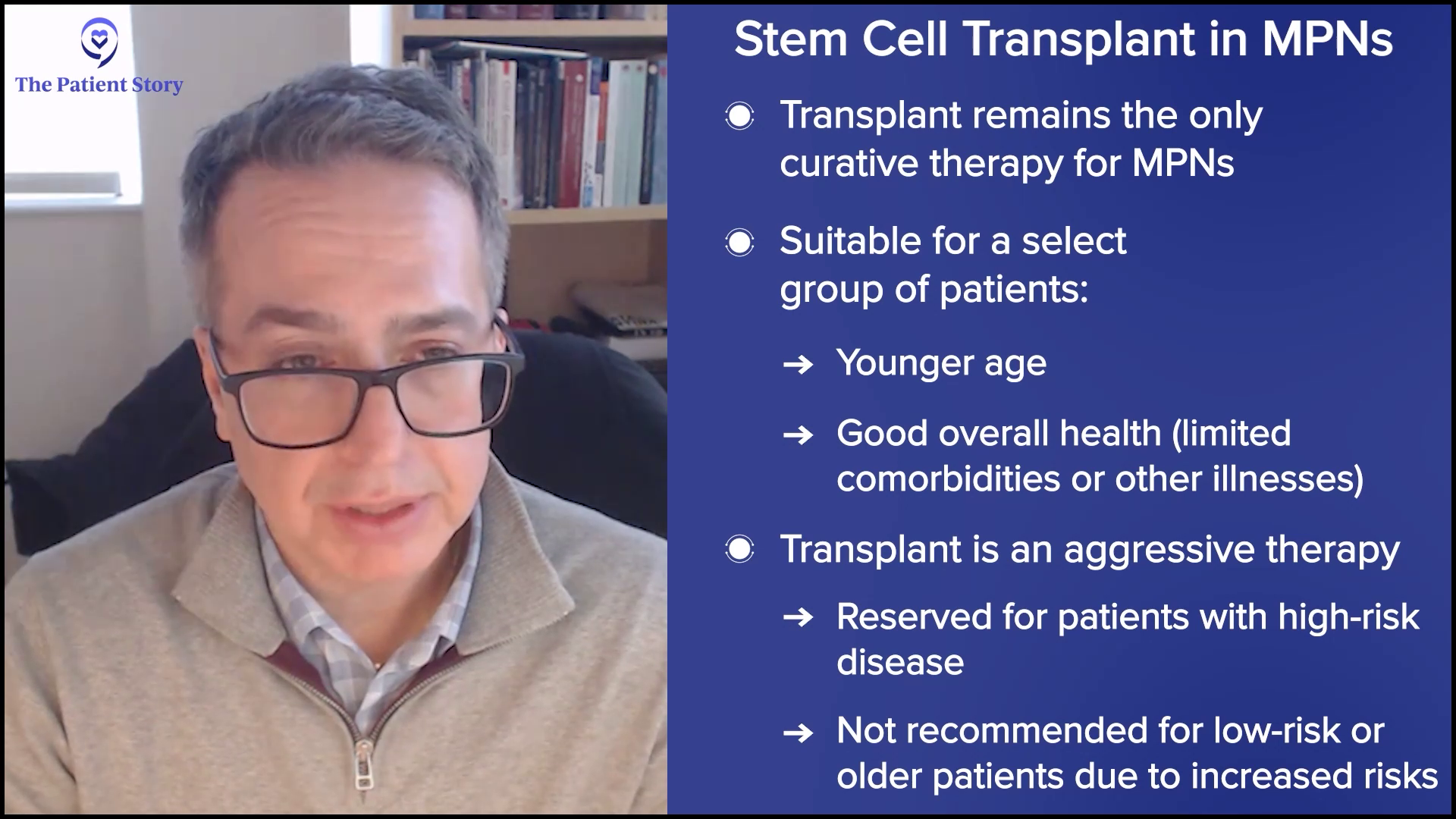
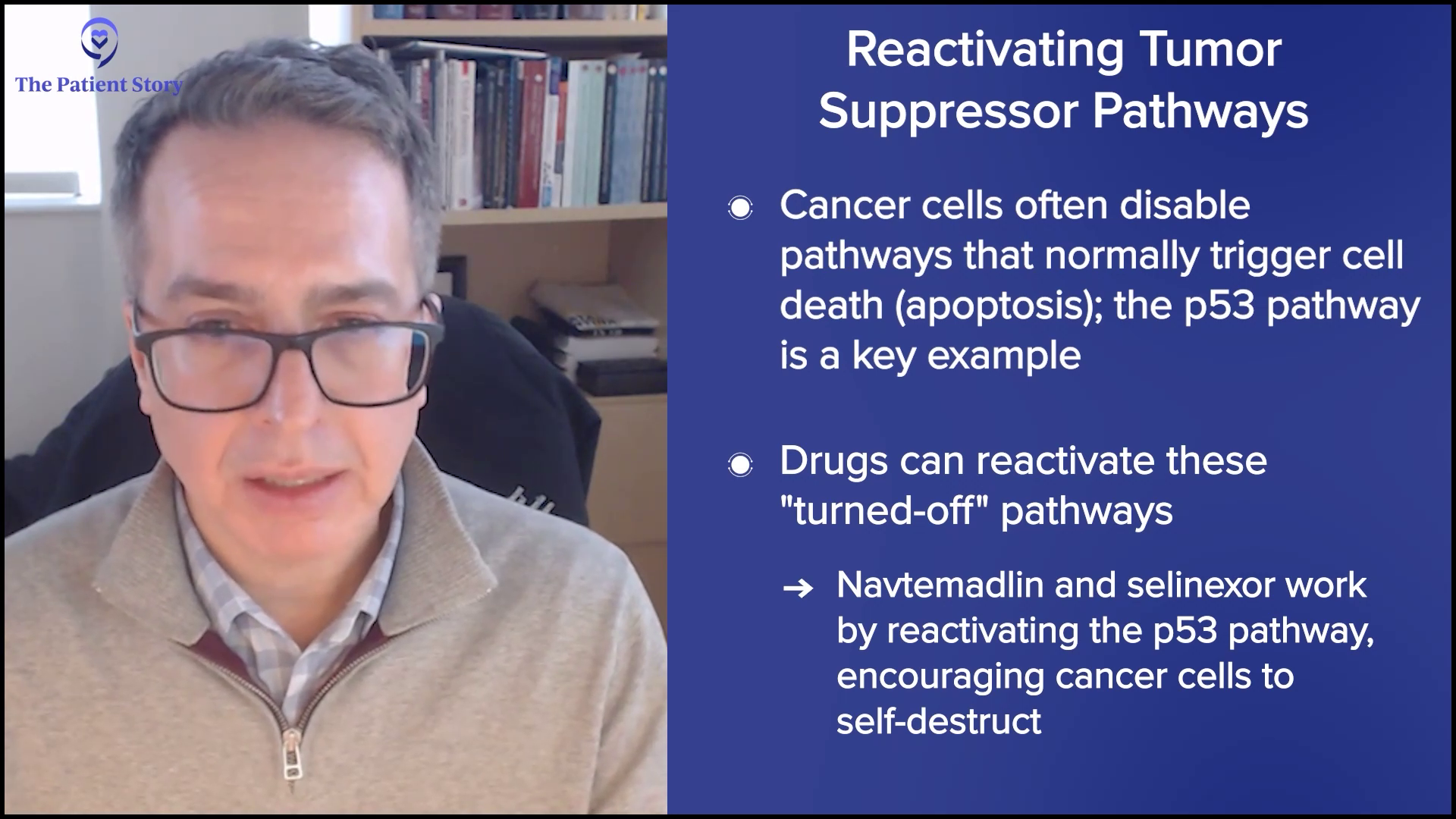
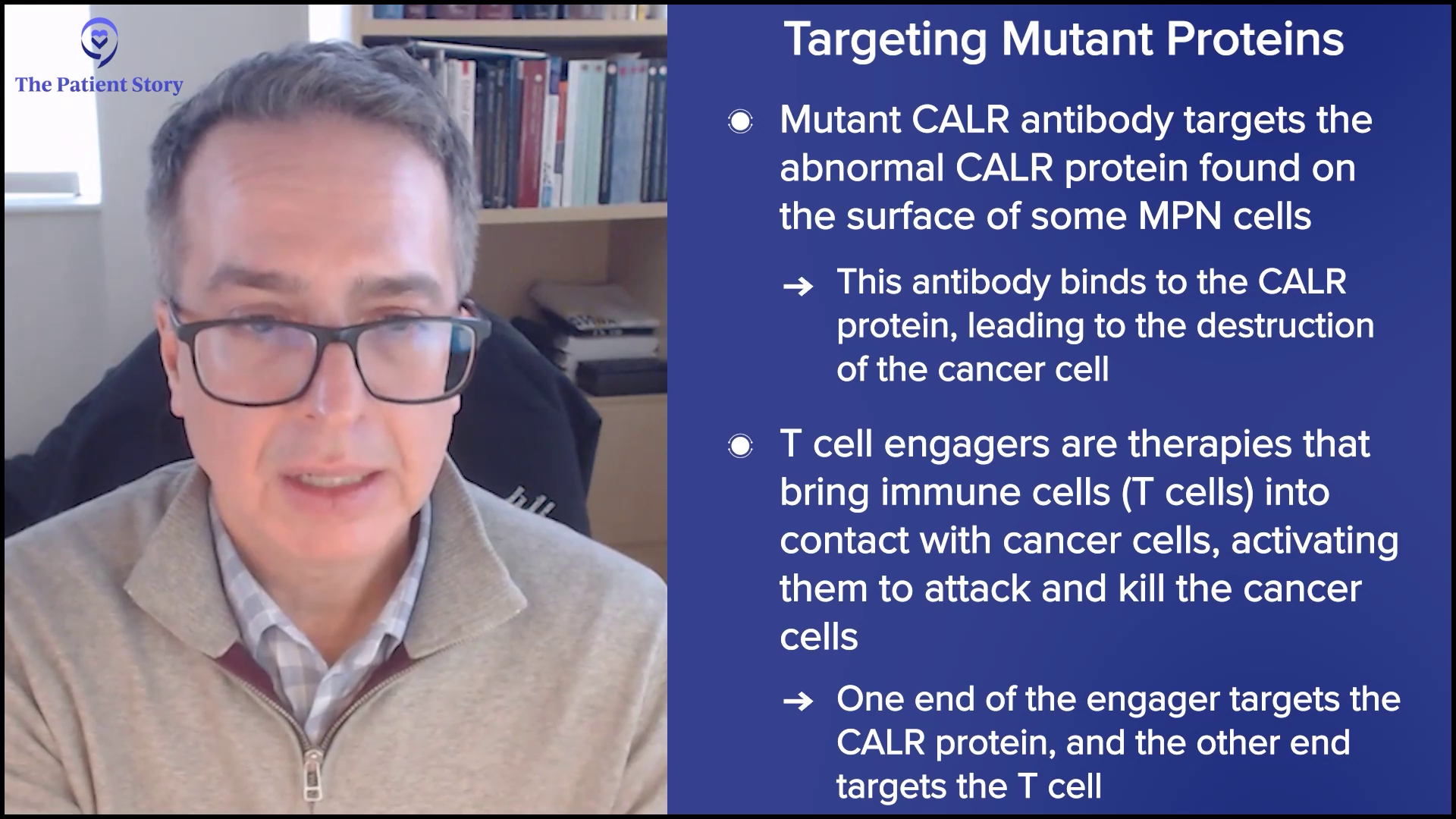
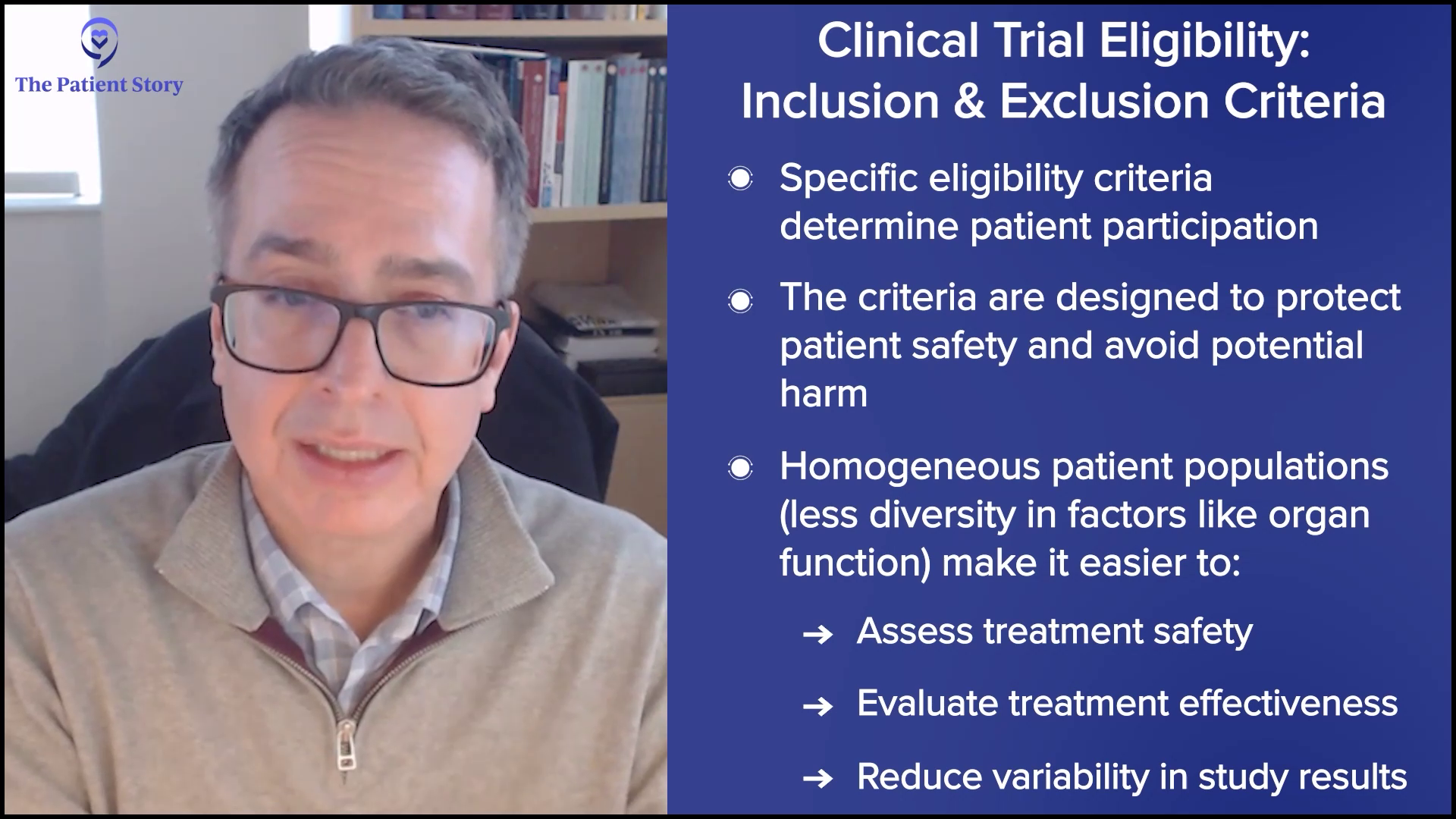
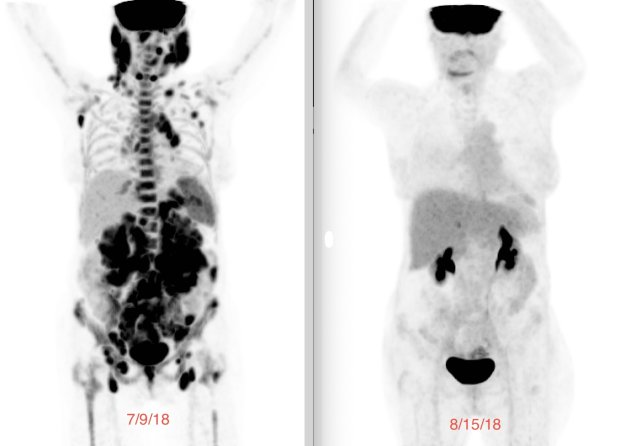
“A Clinical Trial Saved My Life”: Russ’s Acute Myelomonocytic Leukemia (Rare AML) Story
After 48 years of marriage, Russ was blindsided by Acute Myelomonocytic Leukemia (AMML), a rare diagnosis with few options. But a last-minute clinical trial offered a new path. Here is his story of love, surrender, and the “sweet spot” between faith and science.
Interviewed by: Taylor Scheib
Edited by: Katrina Villareal & Jeff Forslund
Russ’s story offers a view into the intersection of honesty, science, and hope. Diagnosed with acute myelomonocytic leukemia (AMML), a rare subtype of AML, after weeks of “just the flu” and crushing fatigue, Russ went from preparing for hospital discharge to learning he had cancer and being told he needed treatment that same night.
When he heard the word leukemia, he thought of death, yet in that moment, a surprising sense of peace set in as he focused on what it was and how to treat it.
The early days were chaotic though. Russ was turned away from one hospital because his insurance plan did not match their funding, forcing him and his family to pivot overnight to UCLA’s emergency department. There, amid what he describes as ground zero conditions in the ER, he endured hours on a hallway gurney, multiple tests, and a 12‑hour wait before finally being admitted to the oncology floor. Even as he grappled with a 7+3 chemotherapy regimen and spent 29 straight days in the hospital, he remembers mostly trying to protect his family as waves of shock rippled through them.

Behind the scenes, his primary care physician and his family were fierce advocates, pushing for answers and ultimately toward a newly developed clinical trial drug tied to his NPM1 mutation. Russ was too sick to participate in the decision, but his wife and adult children did the research and made the call to enroll him, a choice he says “saved my life.” He now calls himself the poster boy for the trial, still taking the pills daily and returning to UCLA for ongoing labs, doctor visits, and bone marrow biopsies.
Today, Russ lives in deep remission and talks about inhabiting the sweet spot where faith and science intersect. He credits his family’s constant presence, the insight of his oncology team, and the existence of a trial drug that arrived just in time for him.
For others with AMML, he encourages surrendering to trusted clinicians, considering clinical trials seriously, learning about your biomarker, and allowing community, spirituality, and modern medicine to work together in the service of one more good day.
Watch Russ’s video or read the transcript of his interview below to dive deep into his story:
- How Russ’s acute myelomonocytic leukemia symptoms first looked like the flu
- How insurance and hospital systems can create major obstacles, and the importance of having advocates who help navigate where to go next to change the course of care
- Why clinical trials are not last-resort gambles—and how exploring them from the start is the reason he says he is alive.
- How trusted loved ones and clinicians are essential partners in making sense of complex choices and next steps
- Russ describes a profound transformation from shock and uncertainty to “deep, deep remission,” learning to live day by day in a balance of faith, science, and gratitude
- Name: Russ D.
- Age at Diagnosis:
- 68
- Diagnosis:
- Acute Myelomonocytic Leukemia (AMML), a rare subtype of Acute Myeloid Leukemia (AML)
- Mutation:
- NPM1
- Symptoms:
- Flu‑like symptoms
- Profound fatigue
- Blood pressure drop
- Shortness of breath
- Treatments:
- Chemotherapy
- Clinical trial drug: menin inhibitor


Thank you to Kura Oncology for their support of our independent patient education program. The Patient Story retains full editorial control over all content.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make informed treatment decisions.
The views and opinions expressed in this interview do not necessarily reflect those of The Patient Story.
- Who I Am
- Our Love Story: Meeting My Wife in 1976
- Knowing She was “The One” and the Secret to 48 Years of Marriage
- Marriage Through Cancer: A Bond Forged in Suffering
- Living with Fear, Gratitude, and “Self-Contained” Feelings Today
- My First Red Flags Before My Diagnosis
- What We Thought During Those Three Weeks Before Diagnosis
- A Primary Care Doctor and Wife Who Advocated Hard
- The Moment Everything Changed
- Telling the Kids and Family in the Middle of the Chaos
- Insurance Rejection and Being Turned Away While Needing Treatment
- We Had to Pivot to UCLA
- First Time Hearing About the Biomarker and Clinical Trial Option
- My Family Made the Clinical Trial Decision and Saved My Life
- Learning to Live Around the Trial Drug Schedule
- The Power of Family Support and My Goal of Being a Father
- How I Think About Clinical Trials Now
- Life on a Clinical Trial: Monitoring, Visits, and Bone Marrow Biopsies
- Having Extra Eyes on Me
- What I Tell People Who are Considering Clinical Trials
- Learning About My NPM1 Mutation
- The Communication Gap Between Doctors and Patients
- Navigating the Future: Labs, Remission, and Living Day by Day
- Living at the Intersection of Faith and Science
- Surrender, Trust, and “Letting Them Bake the Cake”
Nancy and I met in a nightclub back in 1976… and we have been dancing for 48 years.
Russ D., AMML Patient
Who I Am
I’m probably most known for my passion for life. That extends to enjoying living, my family, music, and sports. I’m pretty passionate and can be moved by the things that I love and enjoy.
Our Love Story: Meeting My Wife in 1976
Nancy and I met in a nightclub back in 1976. I went to this nightclub with a buddy. I saw him dancing with a hot blonde while I was standing against the wall with a beer. I said, “Man, if he doesn’t ask her to dance again, I’m going to swoop in.” He went left, she went right, and I dashed right in and put my arm around her. I said, “Would you like to dance?” and we have been dancing for 48 years.


Knowing She was “The One” and the Secret to 48 Years of Marriage
I told her I loved her within two days. I was somewhat bitten by the “love at first sight” cliché, and it proved to be a good choice. We were engaged after two weeks.
Your love deepens as the years go on. It validates your early love, but then your ongoing love strengthens. It’s the recognition that you love her and you’re going to do what you need to do to keep that relationship going. That’s in light of mistakes, challenges, and difficulties, but also a lot of good times, a lot of laughter, and a lot of joyous living.
Your love deepens as the years go on. It validates your early love, but then your ongoing love strengthens.
Russ D., AMML Patient
Marriage Through Cancer: A Bond Forged in Suffering
My family all thought they were going to lose me, and I have a significant place in our family system. Never having gone through anything of this kind, it was such an emotional devastation to my wife and to my children. My wife stayed strong for my children. She was there for them, but she was there for me, too, and she was as determined as I was to see me well. We both felt like we had life to live.
I never winced once about having cancer. I never cried about it. I just said, “Okay, what have we got to do?” and my wife was the same, right there by me. Our love has deepened as a result of that. Love deepens if you allow it to, and our marriage and relationship are in one of the best places they have ever been in our lives. It is a bond created through suffering that is real and absolutely unbreakable.


Living with Fear, Gratitude, and “Self-Contained” Feelings Today
At times, I feel very self-contained. Not isolated, but within myself. I manage the fears and concerns, and I try to live and savor each day. At times, it is a little bit lonely because I want to be brave for my family and my wife. There are things I manage that I do not tell people about. If there were something really dire, I would. But there is this curiosity of a question: how long do I have?
I had a very rare form of leukemia with not a good prognosis, but here I am feeling as good as I could possibly feel. I am grateful for today. I will do the things today that I always do, and I will continue to beat the drum of being encouraging to others and being caring, seeing people, and being who I am. I definitely feel a bit inward and self-contained at times. It’s like nobody knows what I am going through but me. It’s a little tricky.
It is a bond created through suffering that is real and absolutely unbreakable.
Russ D., AMML Patient
My First Red Flags Before My Diagnosis
I had been at a local hospital for two other stays before I went to UCLA. We thought I had the flu. I was on the couch for a couple of days, but I wasn’t getting better. I had a lot of flu symptoms and fatigue. Then I had a real blood pressure drop, which was scary for both of us. We called our primary care physician and she said, “Go to the hospital.” I was in the hospital for the first stint. I’m sure I was a bit dehydrated. They gave me an IV, kept me under observation, ran some tests, and sent me home with no diagnosis.
Over the next three weeks, I knew something was wrong because I couldn’t walk from my car to my house without being exhausted. The fatigue factor was profound. I would lie on my wife’s lap and say, “I am so tired.” I was so down. My primary care physician had me take a blood test, and it came back with my white blood cells at about 40,000. She said, “Get right to the ER.” I went back, and they diagnosed me with acute myelomonocytic leukemia (AMML) at Providence. They diagnosed it, but they do not treat it. They told me I needed treatment that night.
I cannot tell you the sheer shock. It was surreal, but I felt this peace come over me that said, “Okay, we have to deal with this.” Meanwhile, it was like shock waves running through my family, and I was trying to maintain for them. The shock was real, but the main red flags had been flu-like symptoms and serious fatigue that ultimately led to the diagnosis.


What We Thought During Those Three Weeks Before Diagnosis
We didn’t think it was anything like leukemia. We knew something was wrong, but because I had been largely healthy throughout my life, we thought, “There’s something, but we will take care of it.” There was an optimism. It never dawned on us that leukemia was the culprit. Those three weeks were filled with questions: “What’s going on?”
I am a high-energy guy. For me to have fatigue was alarming to everyone. People were freaking out, asking, “What is wrong with Dad?” because I am usually high-energy. Our minds never went to the dire reality we eventually faced — nothing close.
I couldn’t walk from my car to my house without being exhausted.
Russ D., AMML Patient
A Primary Care Doctor and Wife Who Advocated Hard
My primary care physician was very much on top of things and advocating for me. She was in our corner and treated me with what she knew. When the blood test came back with the accelerated white blood cells, she said, “Get to the ER right now.”
It takes a lot to get me to go to the hospital. I tend to be a little bit too chivalrous or stubborn. My wife was also really urging me. She was consulting Doctor Google, researching and reading, and she did not like what she was seeing. It did not take much for her to get me going as well.

The Moment Everything Changed
That afternoon, I had already been in the hospital for three days, and they told me I was being discharged at 2 p.m. I was actually feeling better after they had treated me a bit. I was packed up and ready to go home. Two o’clock, three o’clock, four o’clock went by.
By 4 p.m., I asked the nurse, “We were supposed to be discharged.” She said, “The doctor has not signed the orders yet.” At 6 p.m., the nurse came into our room with a phone. The doctor was on the phone telling my wife and me that I have acute myelomonocytic leukemia and that I needed to get treatment right away by going to downtown Los Angeles and going to USC.
When the blood test came back with the accelerated white blood cells, [my primary care physician] said, ‘Get to the ER right now.’
Russ D., AMML Patient
That moment was surreal and mind-boggling. Nancy was very afraid, and so was I. We’re older, so when I heard the word leukemia, I always thought of death. I asked the doctor, “How long do I have?” It was like going from 0 to 100 — from just living life to, “What?!” A peace settled on me. I cannot say I was fearful. I was more asking, “What is this, and how do we get after it?”
I had no idea at that point that without the clinical drug, there wasn’t much of a treatment opportunity. We were caught flat-footed and knew nothing about it. We have an education now, believe me. My wife was very upset, unhappy, and concerned. We held each other, moved into the discharge process, went home, picked up a couple of things, and drove down to what we thought was USC Medical Center.


Telling the Kids and Family in the Middle of the Chaos
We went to that medical facility, which was supposedly USC, but USC had sold its building to a public medical group. It was not what we expected. My daughters came down, and I hugged them, but I was inside the building trying to get treatment and move through their process.
My wife handled the bulk of communicating with the entire family about my situation. That was brutal. She was a great mom and was there for them, but it was very tough. My youngest daughter was absolutely undone. She was worried I would not be there for her marriage. She had a serious boyfriend and feared I would not walk her down the aisle. She had a lot of angst, and her boyfriend, her fiancé, was seeing this for the first time and was overwhelmed.
My oldest daughter was emotional but very strong. My wife also had to tell my son, and he and I are very close. That was a tough conversation for both of them. The family had initial fears, concerns, and tears, but then it became, “Okay, what have we got to do here?”
When I heard the word leukemia, I always thought of death.
Russ D., AMML Patient
Insurance Rejection and Being Turned Away While Needing Treatment
I was in a back room getting tests, holding paperwork that the Providence doctor had given me. He said, “Show this paperwork and you will get admitted anywhere.” That wasn’t the case. I had to go through all these tests. I spent seven hours in that back room waiting and talking.
At the end of seven hours, a financial manager asked me to come into an area. She said my Medicare Advantage plan did not sync with their public funding, so they couldn’t treat me there. We went from 0 to 100, thinking we would go there and get treatment. We had prepared for that. Instead, she basically said, “You will have to leave. We cannot treat you here.”


I had already released my family to go home because it is about a half-hour drive from where we live. Around 11 p.m., I called my wife and my daughter Danielle and said, “I need you to come pick me up. They’re rejecting me because of the funding.” They asked, “What are we going to do?” I said, “Come pick me up. Let’s get a good night’s sleep if we can, and I will deal with this in the morning.”
I slept well that night, but my wife didn’t at all. In the morning, we called an oncologist whom Providence was going to connect us with. This doctor had never seen me in the hospital, but was on the phone. She said, “Go to UCLA and be really sick, because you are going to have to go in through the ER. Take your paperwork and go through their emergency room.” That is where we headed in the morning.
My Medicare Advantage plan did not sync with their public funding… They’re rejecting me because of the funding.
Russ D., AMML Patient
We Had to Pivot to UCLA
I knew nothing about a biomarker at that point. I was just trying to remember the name of what I had. I was not “AMML-ing” it very well. My oncologist at Providence had told me, “Pretend that you’re very sick at UCLA.”
We arrived and waited in a tent outside in a parking area and it was cold. When I got in, the UCLA ER looked like the ground zero of a battle. There were people everywhere, gurneys, IV drips — everything. I went through a variety of stations, and my wife Nancy and my daughter Ally came with me. At one point, I was really in anguish — or pretending I was — and really doing my best to make myself seem very sick. My daughter Ally asked my wife, “Is Dad really hurting that bad?” I pulled down my mask, looked at her, and winked, so she knew I was not that bad.
They told me they were going to admit me, but didn’t know when. They put me on a gurney in a long hallway with bright recessed lighting. It was like a traffic jam on the freeway — gurney after gurney wrapped around the building. I don’t even know how the doctors and nurses kept track. I received good treatment there; they checked on me, made me comfortable, and did more tests.


We arrived around 10:30 a.m. I was put on a gurney around 1:30 or 2:00 p.m. Eventually, they tucked us into a little side room where my gurney barely fit. It was more private because they knew they were going to admit me. A doctor came in and said, “This is what you have. It’s very treatable. You’re going to be just fine.” That wasn’t true, but we didn’t know that, and we took great comfort in it. She even prayed for me, which was very kind.
We sat in that small room until about 9 p.m. Then we were ushered into UCLA Ronald Reagan on the oncology floor. It took about 12 hours to get a room.
My care practitioner got me settled for the night, started a drip line, and I was attached to that pole for the next six months for the most part. It was a simple “Welcome, get in, and let’s set up what we need.” I told my wife she needed to go home. She was not happy about it. She spent several nights with me over time, though I’m not sure if she spent the night that first night.
They started testing in the middle of the night, so I got very little sleep. Providence had sent all its paperwork, so UCLA had a baseline, but they wanted to run their own tests. They did that through the night and into the next day, and they started chemo on the second day.
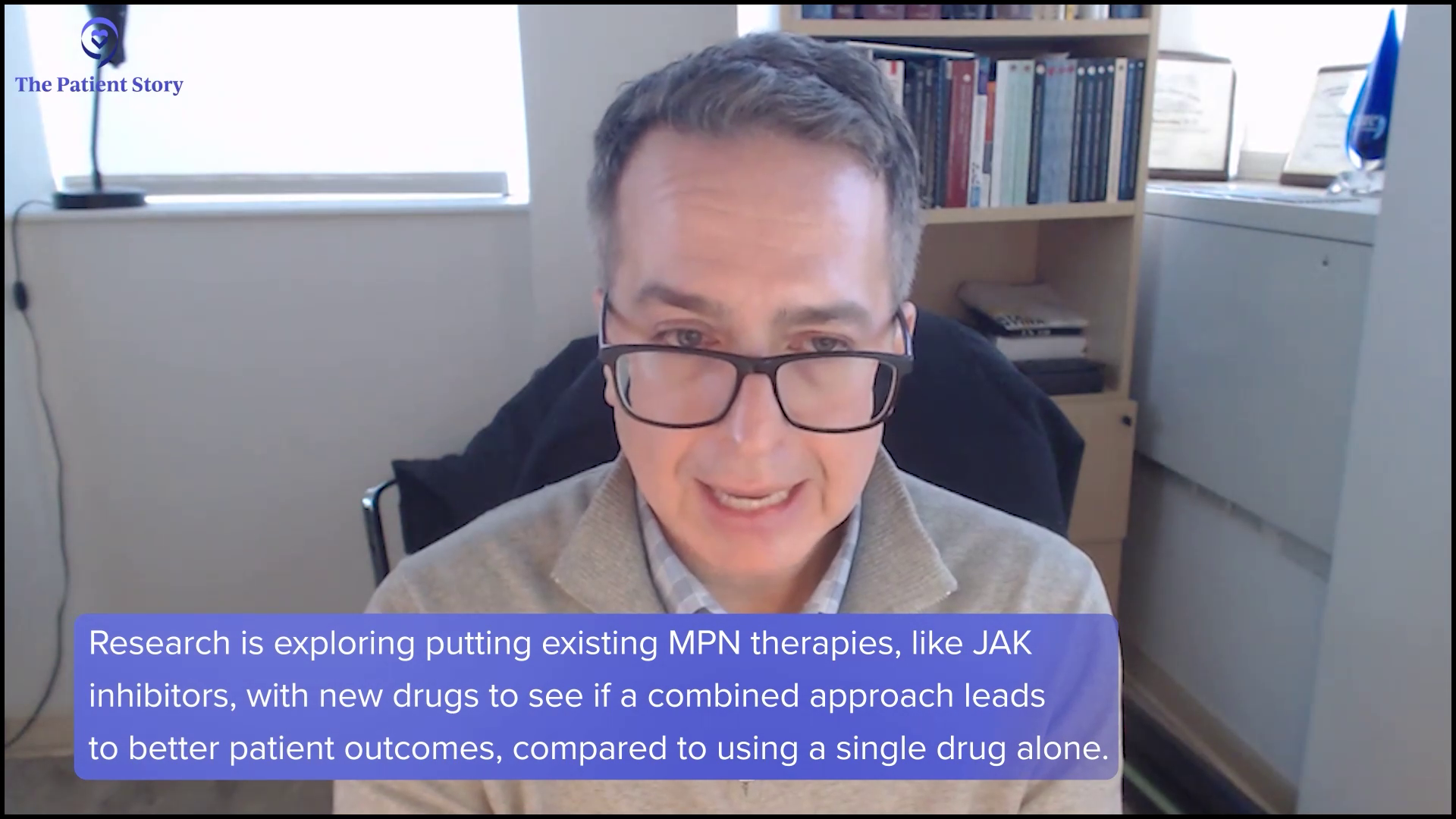
I didn’t know what a clinical trial was or how it would impact me… She said it would be very exciting if I qualified because of the NPM1 mutation.
Russ D., AMML Patient
First Time Hearing About the Biomarker and Clinical Trial Option
My first oncology doctor came to check on me and talk with my wife, my daughter, and me about the possibility of a clinical trial drug, of which I was thoroughly uninitiated. I didn’t know what a clinical trial was or how it would impact me. At that point, I had an IV pole with about 10 different bags running into me. I’m not a big pill-taker, and I had lost control of my life. All these choices were being made on my behalf. I was just along for the ride.
I remember her talking about this drug. I don’t remember her using the word biomarker. She talked about a mutation. That was the first time I heard anything about it. She said it would be very exciting if I qualified because of the NPM1 mutation. That was the first time my family and I heard it, but then nothing more was said for several days, until it actually got in motion.
I was in a bad way, dealing with the seven-day, round-the-clock 7+3 regimen. I had a rocky five or six days. While I was struggling, the clinical drug decision process was going on. My daughter and her husband had done a deep dive into the drug, and my wife had a close family friend with bladder cancer whose life was saved by a clinical drug. Between their research and that recommendation, my family moved toward the trial, even though others initially did not want me to suffer more after hearing about the side effects. My son and my other daughter relented, and we decided to go for it.


My Family Made the Clinical Trial Decision and Saved My Life
They saved my life.
We did not have any other option. This drug had just been developed a few months before my diagnosis, so we believed it was a God thing. If I had been diagnosed a year earlier, I probably would have been gone by now. My prognosis was not favorable. There wasn’t anything else.
I was grappling with trying to get through the chemo. I remember being shivering cold, never able to get warm, and being in and out of sleep with violent dreams. My vision became extremely blurry for a while, which was very scary. I couldn’t focus on anything, which was terrifying. I was wondering if I was losing my eyesight. While I was in that state, my family was discussing the clinical drug. It wasn’t something I could participate in. When they explained it to me, I said, “Fine, let’s do it.”
The two people running the trial came in and handed me the paperwork. I signed it while on my bed. From that point forward, I was taking two pills a day.
We did not have any other option. This drug had just been developed a few months before my diagnosis, so we believed it was a God thing.
Russ D., AMML Patient
Learning to Live Around the Trial Drug Schedule
I tried to get them to give me the pills at the same time each day. You couldn’t have anything in your stomach two hours before taking them and then an hour after. It felt like madness. I tried to get them on a routine: “I will have lunch, you come in at 4 p.m., give me my pills, and I will have dinner at 5 p.m.” It took a week to get them aligned.
At that time, I followed the timing rules very strictly. Now I fudge here and there. They have since told me they don’t know exactly what should be done regarding food intake, because food actually integrates fine with the pills. Waiting two hours before and one hour after may be overwrought. I was very fastidious about it then, though.


The Power of Family Support and My Goal of Being a Father
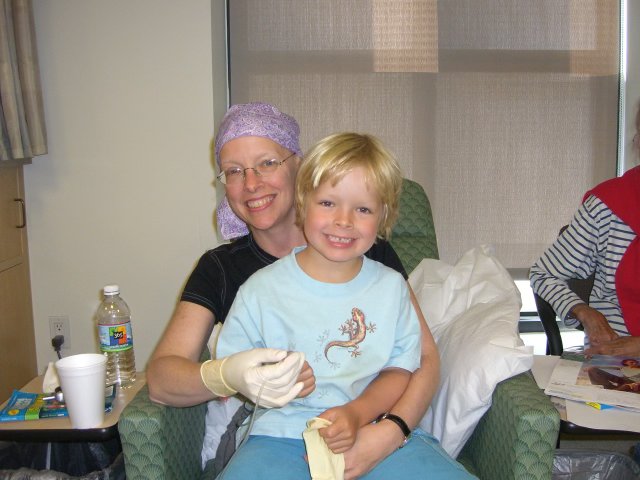
Our family is close. I love being a dad. My wife is an incredible mother. We have always had good relationships with our kids and loved raising them. All my kids and grandkids live within 20 minutes of us, so we’re always together.
I did not grow up with a father who cared about me or even talked to me. There was no connection between us. Becoming a father was a real goal of my life. A lot of people have goals of success. I wanted to be a father. I didn’t know how poor my upbringing was until I became a father myself. I dreamt of being a dad, and it came to pass. The greatest source of joy in my life is my family.
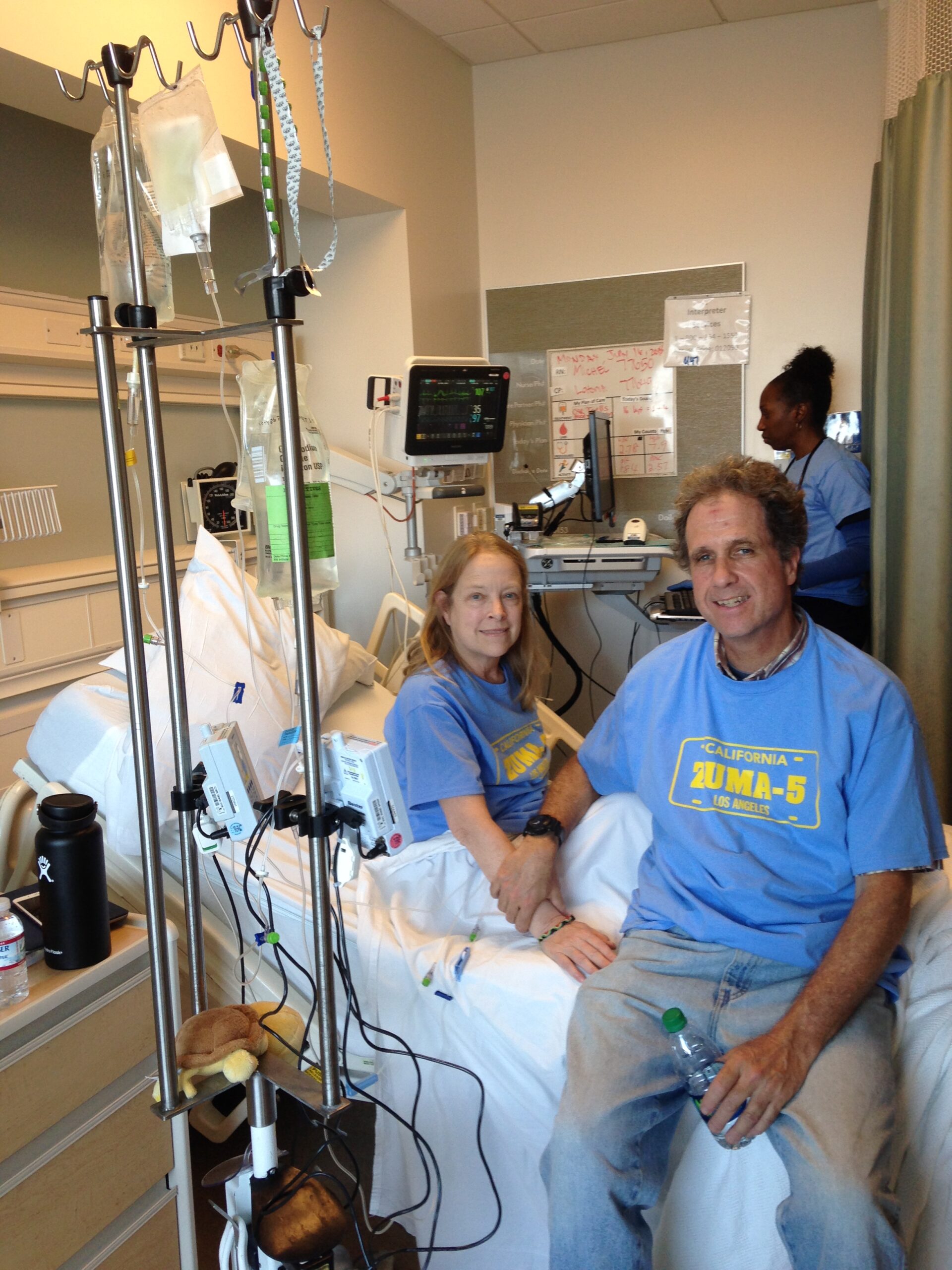
Them caring for me was very humbling. I was at UCLA for 29 straight days during that first stint. My wife, daughters, and son-in-law spent the night with me at different times. I had someone at the hospital every day but one. I was very focused on beating this and having life, and my family was the primary inspiration for that.
Them caring for me was very humbling… I was very focused on beating this and having life, and my family was the primary inspiration for that.
Russ D., AMML Patient
How I Think About Clinical Trials Now
For me, the clinical trial has always been a very positive point of reflection.
I came to understand how rare my cancer was and that there wasn’t much that could be done without this drug. Once we started, I never wavered about being part of it. I felt I was helping, and now a drug related to mine has become FDA-approved. The company that manufactures it has been gracious and kind to me because I was one of the first people to take the trial drug upon diagnosis. The FDA approval initially came for those who had relapsed, but they saw the drug work in me at diagnosis.
I felt it was a real privilege and I was very grateful. I told the company, “Anything I can ever do for you, I will.” I have done some speaking for them. I felt good about helping others, forwarding healing for people coming after me. When I reflect on it, I’m always glad to be part of it and willing to do anything I can to further healing for others.


Life on a Clinical Trial: Monitoring, Visits, and Bone Marrow Biopsies
After being discharged from the 29-day hospital stay, the clinical drug protocol required me to return to UCLA twice a month initially. The first appointment of the month was just labs. The second was labs plus a doctor’s checkup. That went on for seven or eight months. Now it’s once a month at UCLA, where I do labs and see the doctor at the same visit.
I also still get a bone marrow biopsy every 90 days. I just had one about a month ago, and everything came back MRD-negative. We are about six months away from the end of the two-year trial, so something will shift at that point, but I am not sure how yet.
I still take the trial pills. I used to take two pills a day; it’s now three pills a day and has been for six or seven months. I take them in the afternoon. With the first one, I say a small prayer of thanks: “Lord, thank you for [this drug] and that it has become FDA-approved.” With the second, I say, “Thank you for its ongoing efficacy in my body to bring healing.” With the third, I say, “May it always be potent, may I never die of cancer, and bless oncology.”
With the first one, I say a small prayer of thanks: ‘Lord, thank you for [this drug] and that it has become FDA-approved.’ With the second, I say, ‘Thank you for its ongoing efficacy in my body to bring healing.” With the third, I say, ‘May it always be potent, may I never die of cancer.’
Russ D., AMML Patient
Having Extra Eyes on Me
Being in a clinical trial means there are extra eyes on you — extra monitoring, extra visits, and extra biopsies. That hit me early, and I took to it like a duck to water because I felt it was part of my recovery. My wife and I have to drive down to UCLA on a major thoroughfare and fight traffic. It’s not always convenient, but my feeling is that we need to be the best patients, the most pleasant, and grateful people we can be. Let the eyes be on me.
I welcome that because it gives the team more data and information. It can help with this nasty disease. I jokingly call myself the poster boy for this clinical drug because I was one of the first to get it upon diagnosis, so they watch me very closely. I embraced my poster boy responsibilities.


What I Tell People Who are Considering Clinical Trials
If I were sitting in front of a group of people who might know something or nothing about clinical trials, I would start by saying: clinical trials saved my life. I am here today because of a clinical trial drug.
I would encourage you that if there is an available trial drug, do your research, talk with your doctors and caregivers, and seriously look at it as a means by which you embrace treatment that is the absolute best for you. I admit I have a bias; the clinical drug saved my life, so I am a strong proponent. Every person is different, and every situation warrants its own process, so do your process. But if you are going to lean in and need a little help deciding which way to go, I am going to nudge you toward the trial.
Clinical trials saved my life. I am here today because of a clinical trial drug.
Russ D., AMML Patient
Learning About My NPM1 Mutation
The process of discovering the mutation and biomarker was not discussed with me at the time. They did the tests and used the results to guide my treatment.
Later, one of the doctors in the entourage wrote everything down for me on a sheet of paper: the NPM1 mutation, some details, and clarifications. I still keep that piece of paper on my desk. It was the first time I truly understood anything. Up to that point, everything had been verbal, and it did not fully land with real cognition. That paper was a breakthrough moment for me.
It was also the day they told me I was in deep, deep remission. I had never heard of anyone being in “deep, deep remission,” only remission. My oncologist called it “deep, deep,” so I quote him on that. The paper is special because she took the time, on her own initiative, to write it out and make it clear to the degree a layperson could understand.

It was a breakthrough moment for me to understand, and it was also the day they told me I was in deep, deep remission.
Russ D., AMML Patient

The Communication Gap Between Doctors and Patients
It’s important to humanize all of this because there’s so much information being thrown at you. In my case, it was not backwards, but they knew what to do with me. They knew about the clinical trial and my mutation, which was reassuring.
However, there’s a disconnect between the medical field and patients. Medical professionals are so busy and consumed that they look for the shortest paths to communicate and fulfill their jobs. They take for granted that patients will understand information to a certain degree. There are rare doctors who make it simple, and that is genius: having tremendous knowledge but breaking it down so anyone can understand.
A lot of doctors do not seem to enjoy that part of communicating with patients. They may trivialize it because they’re focused on saving lives. That creates disconnect and frustration for patients and families. I’m not judging them; I respect their learning, work, and time. It’s just a reality. What helps is follow-up, like when a couple of nurses came back into my room to help me understand more. That was very valuable.
Genius is tremendous knowledge broken down so anyone can understand.
Russ D., AMML Patient
Keeping lines of communication with the care team open is crucial, but it’s hard for one person to hold all the information. My wife was so focused on me that she did not understand a lot either. My oldest daughter became an advocate, but she eventually had to drop off because she has a family and responsibilities. During that first month, she was all over it by helping explain, fielding conversations, and taking on discussions I couldn’t have because I wasn’t well enough. My wife was too concerned about me to shoulder it all.
It’s a reality that some doctors’ personalities are not geared toward the communicative side. Family members or advocates who can help digest and interpret are incredibly important.


Navigating the Future: Labs, Remission, and Living Day by Day
Now, I can read my labs and know what I am looking at and looking for. I understand the terminology regarding my biopsies. The future is not really discussed. I do not know how to discuss it, which is why I try to savor each day and enjoy it for what it is.
My primary oncologist has said one key thing: every day you do not relapse makes it more likely that you will not. As far as the future is concerned, I live on that. I do not have bad days. I have never been someone who comes home and says, “I had a bad day.” As far as I know, I am totally cancer-free. That is a good day. Whatever else happens is icing on the cake — and I like lots of icing, especially on carrot cake, which my wife is making for Thanksgiving.
Live your life in the sweet spot of faith and science.
Russ D., AMML Patient
There isn’t much dialogue about the future because it is unknown. My oncologist looks at me and says, “Russ, you’re doing good, man. Keep it up.” He is the director of stem cell research and training at UCLA. He did not push me in that direction, even though it would have been natural. He walked with me and watched me. He told me I had options, which many people do not have. He laid out my options but didn’t tell me what to choose. That was up to my family and me.
Living at the Intersection of Faith and Science
I live in the sweet spot of the intersection of faith and hope. You need people praying for you and a community around you. You want to consider spiritual truth to build your faith and strength to fight your disease.
However, do not allow faith to short-circuit your belief in science. A drug was made for me at the right time in my life that saved my life. God blesses great minds with creativity and the ability to do great things for their fellow human beings. Take advantage of that. Where those tools overlap, live your life in the sweet spot of faith and science.


Surrender, Trust, and “Letting Them Bake the Cake”
If I had one main piece of advice for people with AMML or cancer, the word that comes to me is surrender. Place your life in the hands of your higher power — your God, whatever you believe is larger than you and gives life. Surrender to the knowledge and wisdom of experienced doctors.
My primary care physician said to me, “UCLA has the recipe to bake the cake.” I have always trusted that they have the recipe, and they baked a cake for me. Surrender to God, surrender to the doctors, and embrace peace and savor it.
Another hard part is realizing your life is not your own. You have to accept that. If you try to micromanage every detail, you are not going to make it. I was an advocate to a point, and my wife and daughter were advocates too, but a lot happened because we trusted the wisdom and experience of the doctors. You can fight that truth or surrender to it. I surrendered, though it was not always easy.
Surrender to God, surrender to the doctors, and embrace peace and savor it.
Russ D., AMML Patient

Inspired by Russ's story?
Share your story, too!

Thank you to Kura Oncology for their support of our independent patient education program. The Patient Story retains full editorial control over all content.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make informed treatment decisions.
The views and opinions expressed in this interview do not necessarily reflect those of The Patient Story.
More Acute Myeloid Leukemia (AML) Stories
Russ D., Acute Myelomonocytic Leukemia (AMML), with NPM1 Mutation
Symptoms:Flu‑like symptoms, profound fatigue, blood pressure drop, shortness of breath
Treatments:Chemotherapy, clinical trial (menin inhibitor)
Shelley G., Acute Myeloid Leukemia (AML) with NPM1 Mutation
Symptoms: Fatigue, rapid heartbeat, shortness of breath, low blood counts
Treatments: Chemotherapy, clinical trial, stem cell transplant
Joseph A., Acute Myeloid Leukemia (AML)
Symptoms: Suspicious leg fatigue while cycling, chest pains due to blood clot in lung
Treatments: Chemotherapy, clinical trial (targeted therapy, menin inhibitor), stem cell transplant
Mackenzie P., Acute Myeloid Leukemia (AML)
Symptoms: Shortness of breath, passing out, getting sick easily, bleeding and bruising quickly
Treatments: Chemotherapy (induction and maintenance chemotherapy), stem cell transplant, clinical trials