The Future of Lymphoma Therapy: Expert Perspectives After ASH
Treatment News Coming Out of the American Society of Hematology Annual Conference
We’re talking about the two most common subtypes of non-Hodgkin lymphoma: diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma. We recently had a meeting of minds from all over the world at the 2024 American Society of Hematology (ASH) annual meeting, where doctors and researchers present the latest updates on treatments and potential treatments with the most promise.
Explore advancements in non-Hodgkin lymphoma treatments with cancer experts, Dr. Andrew Evens from the Rutgers Cancer Institute and Dr. Tycel Phillips from City of Hope, and Stephanie Chuang, a non-Hodgkin lymphoma survivor and founder of The Patient Story. Dive into the latest research, treatments, and strategies to empower patients and caregivers. Gain valuable insights including promising clinical trial results, breakthrough therapies, and personalized treatment options.
Discover breakthrough treatments reshaping care for DLBCL and follicular lymphoma. Understand cutting-edge combinations like CAR T-cell therapy, antibody-drug conjugates, and bispecific antibodies. Learn to balance effectiveness and quality of life with insights into side effects and survivorship. Access guidance on clinical trials, emerging therapies, and personalized medicine. Hear directly from leading experts about what’s next in lymphoma research and care.

Thank you to The Leukemia & Lymphoma Society for their partnership. The Leukemia & Lymphoma Society is here for you with information about clinical trials, resources, and support.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make treatment decisions.
- Introduction
- Latest DLBCL Advancements in Front-Line Therapy Options
- Ongoing DLBCL Studies in First-Line Treatments
- How Long Until We See These Options? How Do We Choose?
- Questions for Patients to Ask Their Doctors
- Long-Term Benefits/Risks of Bispecifics
- Treatment Side Effects
- Recent Data About Current FDA-Approved Bispecifics
- Bispecifics vs. CAR T-cell Therapy Mechanism of Action
- Cytokine Release Syndrome
- Monitoring Side Effects
- Can CAR T-cell Therapy Cure Some Patients?
- Community Access to Treatments
- Choosing Bispecifics vs. CAR T-cell Therapy
- Latest Treatments in Follicular Lymphoma
- Top Developments in Relapsed/Refractory Follicular Lymphoma
- How Should Patients Interpret the Results?
- Bispecifics and Follicular Lymphoma
- Key Takeaways
- Conclusion
Introduction
Stephanie Chuang: Hi, everyone! We’re talking about the two most common subtypes of non-Hodgkin lymphoma: diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma. We recently had a meeting of minds from all over the world at the 2024 American Society of Hematology (ASH) annual meeting, where doctors and researchers present the latest updates on treatments and potential treatments with the most promise.
Our goal is that you, as patients, caregivers, and partners, walk away with a better understanding of how to approach your own decision-making with your medical team, specifically how to understand the options and balance effective treatment and quality of life.
I’m a non-Hodgkin lymphoma survivor and I’m very thankful that I’ve had no evidence of disease since then. But as we know, so much goes into trying to navigate life after a diagnosis and that’s why I started The Patient Story. We build community through content and education with the mission to help people navigate life before, at, and after diagnosis and, most importantly, to do it in human terms, not medical jargon. We do this through in-depth patient stories, educational discussions, and in collaboration with partners, like The Leukemia and Lymphoma Society.

We want to thank the LLS for its partnership. If you don’t know already, the LLS offers great free resources, like their Information Specialists and the Clinical Trial Support Center, which is staffed to provide one-on-one support in different areas for the blood cancer community.
The Patient Story retains full editorial control over all of this content as always. While we hope that this is helpful, keep in mind this is not a substitute for medical advice. Consult with your team about treatment decisions.

We have an incredible panel of experts to discuss the latest advancements in the field. Dr. Tycel Phillips is a hematologist-oncologist from the City of Hope National Cancer Center in Southern California. He’s an associate professor in the Department of Hematology and focused on treating patients with lymphomas, both Hodgkin and non-Hodgkin. Dr. Phillips lost his grandmother to pancreatic cancer and his mother to breast cancer while he was studying to become a doctor, and I’m sure that informs the way Dr. Phillips practices as a doctor.

Dr. Andrew Evens is a physician-scientist and clinical expert in lymphoid malignancies at the Rutgers Cancer Institute in New Jersey. He serves as associate director for clinical services and the associate vice chancellor for clinical innovation and data analytics at Rutgers Biomedical and Health Sciences. Over the past two decades, he’s been the principal investigator of more than 80 cancer clinical trials from phase 1 through phase 3 studies.
Our audience is cancer patients and the people around them. The goal is to translate for our audience what matters to them. The two of you are dedicated to the patients you see in your clinic and to the research happening in lymphoma. You go above and beyond outside the clinic walls, so thank you so much for that, especially as a former diffuse large B-cell lymphoma patient.

We’re covering two types of non-Hodgkin lymphoma: one is the most common aggressive form, which is diffuse large B-cell lymphoma, and the other is slow-growing or indolent and the most common form of that, follicular lymphoma. We often see a lot of research in these areas. Let’s go into DLBCL first.


Latest DLBCL Advancements in Front-Line Therapy Options
Stephanie: Dr. Evens, what’s been the standard of care for front-line therapy for DLBCL? What’s happening now with front-line therapy options after we have the POLARIX trial where, for the first time in decades, the FDA approved a new option for patients?
Dr. Andrew Evens: It’s evolved and you said it. R-CHOP chemotherapy had been the standard of care for more than 15 years after rituximab broke through around 2005, but it wasn’t for lack of trying. A multitude of randomized phase 3 studies over the ensuing 15 years tried to knock R-CHOP off the top of the hill.
Finally, one at least had some benefit. In a head-to-head study, polatuzumab vedotin replaced the O in CHOP and garnered FDA approval after a phase 3 randomized trial, which was POLARIX, and pola-R-CHP has broken through. Is it used in all patients? That’s open for discussion. It’s FDA-approved for patients who have a certain clinical risk score.

Ongoing DLBCL Studies in First-Line Treatments
Stephanie: Dr. Phillips, ASH is a big conference. What excited you the most about the ongoing studies for first-line treatments, specifically with R-CHOP and novel treatments?
Dr. Tycel Phillips: Surprisingly, a whole section was dedicated to R-CHOP plus X. The POLARIX study invigorated the field to explore more options to improve the front-line setting because, after so many negative studies, we had hit a point where there was very little interest in exploring a way to improve R-CHOP.
In the 2024 ASH meeting, there were a few studies. Venetoclax was added to pola-R-CHP for patients with aggressive B-cell features and this was presented by Dr. Diefenbach. This study looked at venetoclax at a dose of 800 mg given for five days, which indicated a very high response rate across the board, even in those with triple- and double-hit lymphoma, albeit only seven patients. It’s still a very short follow for all of these, but in this situation, a way to move the needle. Some drawbacks were higher rates of neutropenia and grade 5 CRS events. If that were to move forward, there may be some dose reductions to venetoclax because they went up to 800 mg. The question is whether it’s necessary.


Moving along the theme of adding a small molecule to a chemotherapy backbone, there was a study looking at parsaclisib, which is a PI3Kδ (PI3 kinase delta) inhibitor in patients with R-CHOP, which was presented by Dr. Yucai Wang from Mayo Clinic. Surprisingly, this showed a very high overall response rate for the patients enrolled. It’s a small study and didn’t appear to be subtype-specific, so it’s a little bit agnostic. But as Dr. Evens can tell you, the delta inhibitors have become a dirty word in our industry, so how that moves forward will be very interesting considering that for the most part, outside of CLL, these drugs have pretty much been killed off by the FDA.

Additionally, there was golcadomide plus R-CHOP in patients with large-cell lymphoma. This was presented by Dr. Jason Westin from MD Anderson. Again, this compared to lenalidomide, which is built off of, supposedly keeps cereblon in a more closed configuration, so more inhibition of Ikaros and Helios and more T-cell activity than what we saw with lenalidomide. This also had a very encouraging high overall response rate. We can all agree we can’t hang our hats on phase 2 studies anymore, but this one will move forward into a randomized phase 3 trial to try to see if this can improve on the R-CHOP backbone.

Dr. Lorenzo Falchi presented a study with epcoritamab, which is a bispecific antibody, and R-CHOP. Again, a very high overall response rate. We all suspect bispecifics will move forward in this space. There will be a phase 3 study looking at epcoritamab in this patient population.

Lastly, we had the COALITION study, which looked at glofitamab and R-CHOP or glofitamab and pola-R-CHP. Again, a high response rate in the phase 2 studies, very impressive complete response rates, and a high short-term duration of response; but again, more follow-up. As Dr. Evens can attest to, there’s a third bispecific. The OLYMPIA study is looking at odronextamab in this patient population.
It’s going to be a very, very difficult next couple of years teasing out the new standard of care for front-line large cell lymphoma if any of these phase 3 trials are positive. There are several ones that we didn’t even mention that will likely read out in that time frame. We’re in interesting times, but again, there’s a bit of confusion based on the backbone that’s being used — R-CHOP, pola-R-CHP — and the partner drug.
Stephanie: Dr. Phillips, thank you so much for going over all of that. You could characterize that as difficult and confusing because there are so many options now in a space that you’re looking at.
At the end of the day, whatever gets approved will be great for patients and that will be up to the providers, together with patients, to say what’s best for them.
Dr. Andrew Evens
How Long Until We See These Options? How Do We Choose?
Stephanie: Dr. Evens, how long until people could potentially see some of these treatment options? Let’s say they all get approved at some point. How would you decide for individual patients on recommendations for which way to go?
Dr. Evens: It’s a lot to digest and Dr. Phillips did a great job as he alluded to the tip of the iceberg, but it’s confusing. We say it’s a competitive landscape, but through the lens of the patient, it’s fantastic because it means we have a certain standard and a certain cure rate, and we’re trying to do better.
At the end of the day, whatever gets approved will be great for patients and that will be up to the providers, together with patients, to say what’s best for them. We always desire what’s called biomarkers to try to predict personalized therapy. Can we find a treatment or a regimen for particular patients? We’re certainly not there yet but that’s hopefully along with, in parallel, or, at worst, in sequence. We have a regimen approved for a large general patient population. Can we start to figure out who it works in?

To your point, the question always is how effective it is. It’s not just the initial response, but we want it to be stable and stay in remission. How is it tolerated? If it is as good or better tolerated, then that’s fantastic. If there are different or unique toxicities, is it balanced out by the efficacy? Then we think about the cost of care. We want to make sure it can be affordable for patients.
At the end of the day, whatever is approved down the road that builds upon R-CHOP and pola-R-CHP is all good news. Patient by patient, we’ll have to make smart, informed, personalized decisions.
Dr. Phillips: ASH in 2026 and 2027 will be quite interesting.
Dr. Evens: There’s going to be a lot. You know how it goes, Tycel. Most won’t break through, but maybe I’m wrong. As you mentioned, the bispecifics have the best chance.
With the bispecifics, part of the belief in how they will be upwardly mobile is that they’re built off the backbones of monoclonal antibodies, which have revolutionized how we treat patients with lymphoma.
Dr. Tycel Phillips
Questions for Patients to Ask Their Doctors
Stephanie: Bispecifics look like they might rise to the top. Can you share more about why that is? You talked about more personalized medicine. We’re certainly not there yet with biomarkers, but it’s a conversation that’s very hot for patient advocates as well. What guidance do you have for patients to ask their doctors? What can they ask about these different options?
Dr. Phillips: With the bispecifics, part of the belief in how they will be upwardly mobile is that they’re built off the backbones of monoclonal antibodies, which have revolutionized how we treat patients with lymphoma. As Dr. Evens mentioned, the major improvement was the addition of rituximab to a chemo backbone in CHOP. Ideally, if we look at glofitamab, epcoritamab, odronextamab, and mosunetuzumab, they’re still built off that same paradigm. In this sense, we’re encouraging the patient’s immune system to take more part in trying to fight off these cancers with the addition of chemotherapy drugs that are not necessarily immunotoxic.
In that sense, the combined ability and safety of these medications, especially when the patients get a few cycles of chemo first, support their addition to the front-line regimen. It’s that balance. If you have all these different options, we’re going to start going into the weeds and figuring out if there are any things that separate these regimens, like the safety, tolerability, and convenience of giving these to patients.

Is it more convenient to come in for an injection or infusion every couple of weeks or have to take a pill for a certain number of days? We already know that compliance with oral medications is not great. While you may get surveillance and pharmacovigilance on clinical trials, that doesn’t happen outside of a clinical trial setting. Quite frequently, patients will not be as adherent to these medications as they should be and that may impact clinical outcomes.

Echoing Dr. Evens again, it’s an exciting time. Another part we also want to look at is how they may impact certain subsets. With POLARIX, there’s the issue of subtype specificity, but do we have other treatments that may be agnostic to subtype? If we start getting more of these molecular tests that will divide patients into certain subtypes, how do these treatments broadly apply across the board? Do we need to play mix and match based on a person’s genomic profile? We’re still away from that, but that’s a place where everybody is trying to get to where it’s more individualized for the patient versus everybody who walks in gets the same treatment even though they’re not the same.
Dr. Evens: Dr. Phillips summed it up greatly. Clinical trials can be overwhelming. There’s not a better or the best clinical trial. To me, they’re all good options and that is one thing I would ask your doctor. What’s currently FDA-approved for my condition? Are there any new treatments? For newly diagnosed, there are very mature clinical trials taking the standard of treatment, like R-CHOP or pola-R-CHP, and trying to do better and looking at safety.
Long-Term Benefits/Risks of Bispecifics
Stephanie: There are clinical trials where we’re studying all these possibilities and then there are the approved options. The Patient Story set this conversation up backward in terms of clinical trials in that much of it is done for relapsed/refractory patients first and then works its way up to earlier lines of treatment.
We’re going to talk about bispecifics for relapsed/refractory patients. Dr. Evens, you talked about bispecifics rising to the top. Is there anything else in terms of long-term benefits and risks for what we understand so far when it comes to everything presented at ASH?
Dr. Evens: What about after treatment? Survivorship is very important. Yes, we want to cure with minimal side effects and fairly cost-effective treatment, but there are late effects that remain to be determined. Since these agents are not classical cytotoxic chemotherapy or radiation for the most part, the hope is that they’re going to have less late effects, whether it’s cardiac, pulmonary, or infections. But at the same time, we can’t assume anything. It needs to be studied.
My hope is that, whether through the pharmaceutical companies or academic institutions, it will be bolted on in some form or fashion to these studies to follow clinical trial participants for late effects whether it’s five or 10 years later because we certainly don’t want to cause harm down the road.
Dr. Phillips: When you have options, you could be a little bit more stringent about what’s the best thing moving forward. The more options there are, the better it is for our patients.
Dr. Evens: Tycel, I’m sure you have survivorship expertise at City of Hope.
Dr. Phillips: Yes, there is a survivorship clinic.

Dr. Evens: In the past, I wouldn’t say we minimized it, but maybe we didn’t appreciate or have the science associated with survivorship after treatment, so that’s also a valid question for your oncologist whether it’s two years or five years later. Can we talk more about my individualized survivorship? In most academic centers, even in some non-academic centers, there is survivorship expertise. Whether it’s led by nurse practitioners or physicians, it’s all good as we get into that important part of the rest of life.
Dr. Phillips: Yes, life after chemo.
Stephanie: I can’t tell you both how much I appreciate that you brought that up. I love how survivorship is becoming much more of a focal point. I went through hundreds of hours of chemo. I had dose-adjusted R-EPOCH in 2017 and the thought of being able to focus on survivorship and the things that I need to look out for because my body went through this is wonderful.

Dr. Evens: You look great and I’m hopeful you’re doing well, but it’s something to bring up. I don’t want to make it seem like it’s an epidemic or malady of late effects. They’re slight and subtle, but they’re real and we’re still learning a lot about them. We’ve learned a lot from Hodgkin’s lymphoma, which was one of the first curable cancers in man that we learned in the 1970s. But a lot of the medications, in particular anthracyclines, the vinca alkaloids, the alkylators they’re called, whether dacarbazine or cytoxan, are very similar, so they’re probably more similar than not and, frankly, regardless of age.

One other important pin to put in this point is when we hear about late effects, we sometimes think that’s 20 to 30 years down the road. What we’re learning, at least in Hodgkin’s and it’s probably true in large cell lymphoma if we take a close look, is within a few years and for sure by five and 10 years after treatment, we’re already seeing some of these late effects. It’s important to get that understanding from your local oncologist or survivorship expert.

Dr. Phillips: With anthracycline, a lot of times, we didn’t look and when you looked, you’d see a lot of cardiac effects, even within a year after completion of treatment.
Dr. Evens: You do. Now, it’s always tough. What do you do about it? But at a minimum, controlling your risk factors, like hypertension and cholesterol, certainly can’t hurt. It’s only going to do the body good.
Dr. Phillips: A lot of sites have cardiologists now that have taken an interest in oncology-related events. There are a lot of processes and steps, cardiac rehab per se, that have helped ejection fractions for a lot of patients.
Dr. Evens: There’s more funding. I’m working with a cardiologist at Tufts Medical Center who’s going to Beth Israel in Boston. She’s a cardio-oncologist and got a big grant funded to look at some cardioprotective agents early on, so thankfully, hopefully, they’ll be some resources.
It’s always hard, Stephanie. Whenever we talk about doing research, as you can imagine, you need funding and there tends to be a lot more from the pharmaceutical companies, which is understandable with the medications. With survivorship, there aren’t as many companies there, so we rely on the government through the National Institutes of Health or National Cancer Institute and philanthropy. There might be some other ways, but it’s such an important part of cancer care.
Stephanie: A hundred percent. Let’s get more funding for studying survivorship because that is so impactful for people.
With various agents, there’s always a small risk of secondary cancers that come from chemotherapy drugs. Whether you receive radiation or not is also a very important concern.
Dr. Tycel Phillips
Treatment Side Effects
Stephanie: Are there other late-term effects that people should be thinking about if they’ve undergone chemoimmunotherapy? Based on what you’re talking about from the R-CHOP plus X from ASH, is there anything else to call out specifically? People might think you’re adding a new drug, so does that mean they’re going to experience more side effects?
Dr. Phillips: It depends on the drug. With various agents, there’s always a small risk of secondary cancers that come from chemotherapy drugs. Whether you receive radiation or not is also a very important concern. We’ve learned long-term that some radiation risk factors don’t plateau and rather age with the patient. Those are all things to keep in consideration with some of these other drugs.
Thankfully, even with vincristine, which is a microtubule inhibitor, the biggest thing is neuropathy. The risk is the nerve damage is unpredictable and you may not get a resolution, so there’s always that concern and trying to prevent that from happening.
With some of the newer drugs, depending on their class, they all have unique side effect profiles. In the front-line setting, they’re given for a more limited time, which would hopefully prevent some of the side effects that we see in the relapsed/refractory setting, especially for drugs that are given until intolerance or progression, which is always a concern.
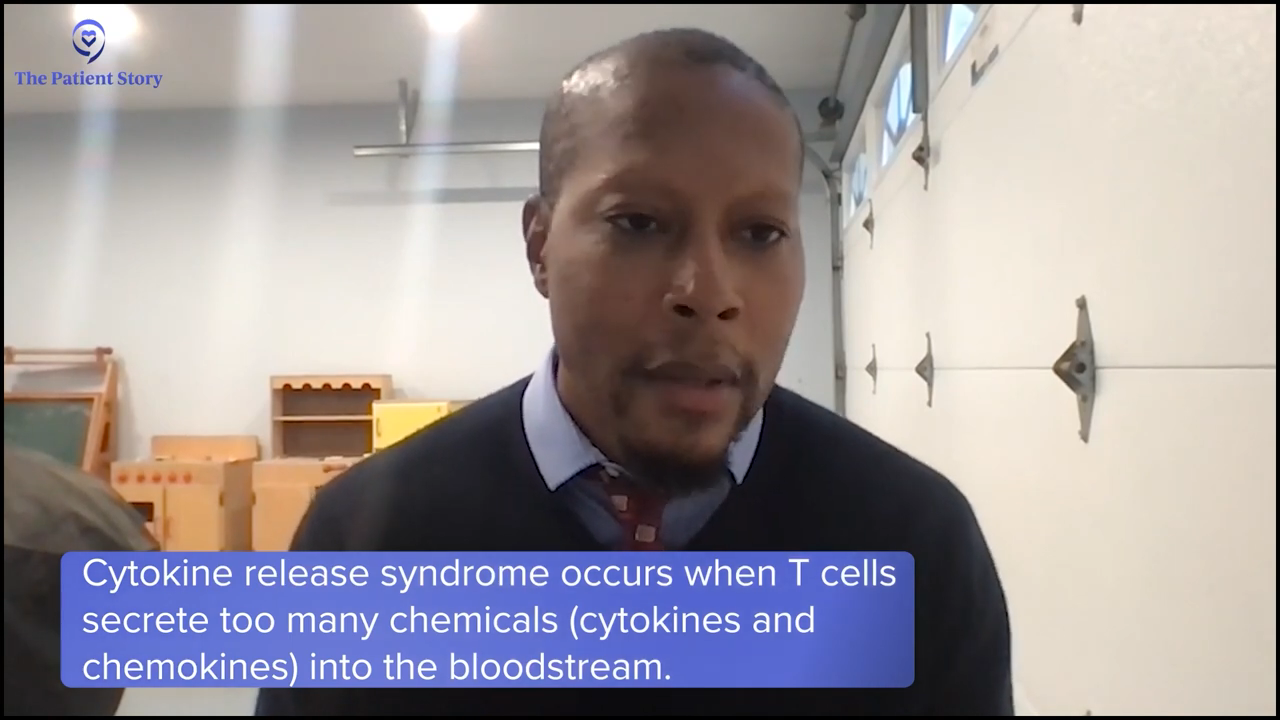
Looking at delta inhibitors in the relapsed/refractory setting, with indefinite use, you worry about liver irritation in some situations, diarrhea, and infection risk. With golcadomide, which is like a lenalidomide analog, reproductive risk is always a concern with that class, so if you’re a young woman, that’s always a concern in handling that medication. It can cause neutropenia, which may increase your infection risk when given with chemotherapy.
With the bispecifics, cytokine release syndrome is the biggest concern. Depending on when you give the chemo, you may mitigate a lot of higher-grade CRS, so you can potentially make that a bit easier to tolerate and less risky if you give a little chemotherapy to debulk the tumor and maybe blunt the immune response a bit.
Recent Data About Current FDA-Approved Bispecifics
Stephanie: Let’s move to relapsed/refractory DLBCL. There are two approved bispecifics for DLBCL: glofitamab and epcoritamab. Dr. Evens, can you talk about the most recent data? What’s relevant to patients about the two of them and the differences?
Dr. Evens: Generally speaking, it dovetails off of a lot of what we’ve already said. They’re very active. There are side effects, thankfully less than CAR T-cell therapy but similar, which is called cytokine release syndrome. Patients experience almost flu-like symptoms and some neurologic side effects as well. Thankfully, it’s less common, but it can still happen, so there certainly needs to be caution.

When we’re meeting a patient who received front-line therapy, whether it’s R-CHOP, R-pola-CHP, or dose-adjusted EPOCH and there’s a relapse, the historic standard was an autologous stem cell transplant. That’s gone down, but not away, with CAR T-cell therapy, commercial CD19.
I know your question was about bispecific antibodies, but there is a multitude of new CAR constructs. The current commercial CARs are CD19. More often than not, that’s the treatment of choice for many patients who relapse the first time, especially if it happens within 12 months, because bispecific antibodies for diffuse large B-cell have been after that. Not that it would be wrong to do it instead of CAR T-cell therapy; we just haven’t seen as much of the data. In some ways, that’s where bispecifics have tried to leapfrog and go to the front-line setting.

Whether it’s used in second-line or third-line, the bottom line is both medications are very active. They’re more similar than different, although there are some key differences. One is given intravenously, the other is given subcutaneously, and one has a few more doses than the other, but they’re largely much more similar than they are different.
Tycel, you don’t ever use standard before CAR, do you, in someone who can take CAR, right?
Dr. Phillips: That was a bridge.
Dr. Evens: Are you doing bispecifics as a bridge to CAR sometimes?
Dr. Phillips: Only in patients whose diseases rapidly progress and we need to stabilize before we can get them to the CAR product. But I agree with you. CAR equals cure. With bispecifics, we guess, but we don’t know for sure. Until we can be 100% certain, you’d be very hard-pressed to put a bispecific before a CAR in the second-line space.
Dr. Evens: Part of that is time. CARs have a several-year lead time and to say “cure,” two years in remission is a pretty good point. After five years, you feel good. That’s where we see CAR T-cell therapies. We saw some data at the meeting settling out around the 30% to maybe 40% range. We’re hopeful that we can say cure. We don’t have that lead time with bispecifics, although we’ll see over the next couple of years where those land.
Stephanie: CAR T-cell therapy came out first in research, so there’s more data and real-world evidence about how they perform. With clinical trials, it’s a different population, so it’s nice to be able to see what happens.
Dr. Phillips: CAR T-cell therapy has remained reproducible in the real-world space as it has in clinical trials, which is not the same as a lot of other drugs where you typically see a decline in efficacy once you move out of the clinical trial space. That hasn’t happened with CAR T-cell therapy.
Dr. Evens: Clinical trials are real world. Real world is a lingo that we use, meaning all comers, regardless of kidney function, age, etc., and not like the qualifications you need to join a clinical trial. He’s exactly right.
To put an extra point on that, for CAR T-cell therapy and bispecifics, there’s almost no upper age limit. With autologous stem cell transplant, you can do it up to patients in their 70s, maybe up to 80, but it gets a little tricky. Not to say CAR T-cell therapy and bispecifics in older age groups are a walk in the park, but they’re very manageable and doable.
Another part of science we hope to understand: which treatment works in which type of patient.
Dr. Andrew Evens
Bispecifics vs. CAR T-cell Therapy Mechanism of Action
Stephanie: Dr. Evens, could you summarize the mechanisms between CAR T-cell therapy and bispecifics in a way patients can understand?
Dr. Evens: It can be confusing for sure. I would frame them both under the rubric of immunotherapy. We talked about autologous stem cell transplant, which is also a fancy word. It’s not like getting a new liver or kidney; that’s called an allogeneic. Autologous transplant is one cycle: a very high-dose chemotherapy blasting hopefully the lymphoma away. But what about blasting your cells? We know that one of the most powerful anti-cancer agents is your immune system — your T cells in particular. We don’t know why, but in some cancer patients, lymphoma patients, they’re not doing what they’re supposed to.

CAR T-cell therapy cell is filtering out your T cells, sending them to a company, and over about a 3 to 4, sometimes five-week period, they’re re-engineering it by doing two things. They’re transfecting an antibody to tell it where to go when you put it back in and then in some ways, you supercharge it or activate it. When you re-inject it, it’s a one-time treatment several weeks later. It not only rapidly expands, but hopefully, it goes this supercharged T cell with the CD19 attached to it wherever the lymphoma is in the body.
What are bispecifics? It’s similar in concept in that it’s the patient’s immune system, but it’s an off-the-shelf treatment. The way to think about it most simply is a double antibody. It’s a CD20, which interestingly is rituximab. If rituximab in most patients stops working, why would this? Because the second antibody is CD3. When it’s off the shelf and injected, CD20 goes to where the lymphoma is, but the CD3 has T cells attached to it, so it delivers the patient’s endogenous or in vivo T cells to where the lymphoma is.

It’s remarkable. I’ve had a multitude of patients, as I’m sure Dr. Phillips has, where CAR T-cell therapy is given, it doesn’t work and we don’t honestly know why, but we’re thrilled when it happens, then you give bispecifics, and the lymphoma completely goes away. This is someone who has already failed regular CD20 single antibody, CD19 CAR, and then a different immunotherapy for one reason or another is effective. That’s another part of science we hope to understand: which treatment works in which type of patient.
Stephanie: There’s a lot of science to wrap up into this very abridged version, but I know the goal here is this more targeted. It’s engineered and using your immune system and it’s targeted with the goal being that we feel fewer side effects than blasting the whole body with chemoimmunotherapy. Dr. Phillips, is that how people can think about that?
Dr. Phillips: When I try to find the easiest way to compare chemotherapy to other treatments, I go back to the military where chemo is like napalm. It’s going to kill anything that’s growing rapidly because that’s what it’s designed to do. It isn’t specific for anything, one way or the other. A lot of these targeted treatments, such as bispecifics and CAR T-cell therapy, are more focused on directing your treatment toward the problem, which is, in essence, a malignant B cell.
There is still some ambiguity because normal B cells will be impacted. After all, we’re still targeting CD20. Hopefully, in the future, we’ll have some cancer-specific markers that may even spare normal immune cells. But to this point, we’re sparing a lot of other tissue because it’s directed toward a malignant B cell, which chemotherapy can’t do.
The closest we can get to that is antibody-drug conjugates, which will deliver a toxic drug directly to the tumor. The drug isn’t active until it’s internalized and then it can kill the tumor off. But even in that sense, you will get some spillage. As the cancer cells die and open up, that drug is also free to kill other bystander tumors but also any normal tissue that’s in the area, which explains some of the side effects we see.
Stephanie: I love it when there’s real-world verbiage so people can understand better.
Cytokine Release Syndrome
Stephanie: Dr. Evens, cytokine release syndrome was mentioned as a monitored side effect when it comes to immunotherapy. What does that look like in patients and what are the differences between what you’ve seen in the responses with CRS from CAR T-cell therapy versus bispecific?
Dr. Evens: It’s very similar as to when it happens. Thankfully, with bispecifics, it’s usually less frequent and less severe, although not zero, so you still have to have some caution. We don’t like to talk about it, but whenever we mention grade 5, that’s a fatal side effect and thankfully, it’s very uncommon, but we want to keep that 0%, as long as there’s the right training and the right medication on the shelves.
When I say the right training, it’s not the oncologists, doctors, nurses, and pharmacists. It’s making sure your emergency room and intensive care unit know. Like for many things in life, you’re preparing for the worst in case it happens.
For example, I gave a patient a bispecific antibody on a Friday. By Wednesday night, that patient had a very high fever, so he was experiencing a delayed cytokine release. He went to the emergency room. We have a protocol, so they know what to look for. We had a conversation and were able to mitigate it. The patient did not need to be admitted to the hospital. The fever quickly came down and he felt fine.
It’s a team sport as we’re managing some of the side effects and thankfully, almost always, it’s very manageable but it’s important to have that communication and collaboration across all specialties.
If a patient has cytokine release syndrome, the earlier you intervene, the better because you can mitigate and stop it from escalating to a higher grade, but your team has to be aware of what to look out for.
Dr. Tycel Phillips

Monitoring Side Effects
Stephanie: A huge topic for patients is the logistics side and considering what they have access to. Dr. Phillips, can you paint that picture for those considerations when it comes to patients? Do they have to be monitored? How long do they have to be monitored for potential CRS after CAR T-cell therapy and bispecifics? How can that impact the choices that they may have depending on where they live?
Dr. Phillips: In essence, CAR T-cell therapy is a little different. We typically think that the patient will be in and around the cancer center for about 30 days. You also are required to have a caregiver with you almost 24/7. Even in institutions where we can move patients to outpatient for CAR T-cell therapy, that still requires you to be on campus, have a caregiver, and go back and forth to the hospital. It requires more logistical hurdles to get over.
In some sense, CAR T-cell therapy is also akin to transplant centers. A lot of these things are in place as far as the inpatient portion because instead of admitting somebody for high-dose chemotherapy and then a stem cell rescue, you’re giving them CAR T-cell therapy with inpatient monitoring and very close follow-up. When you’re in the hospital, they’ll be monitoring you for neurological changes and vitals will be taken to monitor for any signs of cytokine release syndrome. These are done very closely.
Bispecifics, on the other hand, as Dr. Evens has eloquently elucidated, can be given in a community setting. The only real thing you need to have is education for the patient and the staff about what may happen if bad things happen. If a patient has cytokine release syndrome, the earlier you intervene, the better because you can mitigate and stop it from escalating to a higher grade, but your team has to be aware of what to look out for.
Normally, when a patient is on any oncologic treatment and they come in with a fever, you reflexively think it’s an infection and you give antibiotics and draw blood cultures. You don’t necessarily think cytokine release syndrome. With bispecifics, you need to think of cytokine release syndrome. Even if you are working up for an infection, you need to be putting those things in place to mitigate cytokine release syndrome.

Having certain medications on the formulary, like tocilizumab, can be difficult because it is cost-prohibitive, to be honest, so every hospital system doesn’t want to keep this on, especially if they’re not using it. These need to be in place. Most centers that have incorporated bispecifics have a designated champion, which is somebody who plays quarterback to organize the rest of the team. We have plans in place when patients come in. We have contact information and if anything goes awry, they’re there to fix and get everybody realigned, so when the next patient comes in, it doesn’t happen.
The timing depends on what you receive. IV medications tend to have an early onset of cytokine release syndrome with bispecific. With these subcutaneous formulations, sometimes these things happen at home, at which patient education becomes very important.
Make sure you have a thermometer at home to monitor for fevers. Some people have blood pressure monitors. Some people have advocated for other things to self-help. Make sure you have antipyretics at home, like acetaminophen or ibuprofen. Some physicians send patients home with a pill in a pocket, which is steroids, which in some situations can mitigate cytokine release syndrome or at least keep things stable until they can get to the hospital.
Can CAR T-cell Therapy Cure Some Patients?
Stephanie: Dr. Evens, you mentioned the word “cure,” but we need data. There was the five-year data for ZUMA and TRANSCEND trials. From a quick glance at that, is there anything more you want to add to this general feeling about CAR T-cell therapy and its ability to be curative? I know you talked again about needing more time.
Dr. Evens: It is time, though, Stephanie. It comes down to that. In the pre-CAR era, we tell patients that hopefully it doesn’t come back. In the minority of cases when it does, it most commonly happens in the first two years and then a little bit through years three, four, and five. It’s uncommon after five years with chemotherapy, but never impossible.
We expect that that dynamic should be the same because it is an aggressive cancer. How can it live around for five-plus years? These numbers we’re seeing at five years is what we call the plateau in the curve. It looks pretty darn flat once you’re getting out to three, four, and especially five years. I feel pretty confident we can use the word cure.
Anytime you have a treatment primarily anchored around academic medical centers when we know that over 80% of cancer care happens at nonacademic centers or community centers, there’s going to be a problem and a problem related to access.
Dr. Andrew Evens
Community Access to Treatments
Stephanie: Dr. Evens, widely discussed in patient groups is accessibility to CAR T-cell therapy. You both are at major academic centers. You’ve got all the tools and the latest, but for a lot of people who live in the community, this doesn’t seem to be very feasible.
Dr. Evens: That’s a major issue and Dr. Phillips aptly alluded to this. Depending on which data set you look at, in some cases, only 20 to 25% of patients who should receive it are receiving it. It will never be 100%, but it certainly should be more than 20. Anytime you have a treatment primarily anchored around academic medical centers when we know that over 80% of cancer care happens at nonacademic centers or community centers, there’s going to be a problem and a problem related to access.

What are ways to circumvent that? We don’t want to do anything haphazardly with any treatment. Can we try with certain community centers in collaboration with academic centers or maybe on their own and working with pharmaceutical companies or even foundations to be able to do it? You bet. In New Jersey, some strong, non-academic medical centers are giving CAR T-cell therapy carefully, so it definitely can be done. Better knowledge and prophylactic treatments, like steroids or tocilizumab. There was some data on this at the 2024 ASH meeting.
Maybe we can get to second- and third-generation CAR T-cell therapies that are engineered in a way that rapid overexpansion of T cells, which is driving a lot of the side effects, is more predictable or doesn’t happen. I’m hopeful that we’ll get there, but that’s part of the reason why bispecific antibodies have become a little easier.
Assuming the patients have access, some can’t wait four or five weeks. They don’t have enough time. It’s a big issue, but it’s top of mind and we’re thinking about all of these issues and how to remedy them. You guys have community centers don’t you, Tycel, not just in Duarte, right?
Dr. Phillips: We have a lot of community sites. They’re not doing CAR though.
Dr. Evens: They’re not? You know what we’re starting to do? We’re segmenting it. We loosened it to a little less than 30, but what we’re doing is having them do the pheresis. We have the New York Blood Center. It’s going to be tough with the current commercial CAR. Maybe in a bigger community, but it’s going to be tough in a small one.
We’ve had several data sets that show that you can still successfully harvest and administer CAR T-cell therapy in patients who’ve been exposed to bispecifics.
Dr. Tycel Phillips
Choosing Bispecifics vs. CAR T-cell Therapy
Stephanie: With bispecifics being more accessible in the community and in terms of wait time, when would you want to give bispecifics versus CAR T-cell therapy? Let’s say a patient has both as an option.
Dr. Evens: We’re not sure bispecifics are as effective as CAR T-cell therapy in the second-line setting. Let’s say for one reason or another, you can’t get CAR T-cell therapy. I don’t know what you think, Tycel. It wouldn’t be wrong to give bispecifics.
The only mild note of caution would be it’s not like rituximab. Even though most patients do well, you have to have some structure and organization in place before you even give the bispecific antibodies. They’re more doable for community oncology for sure than CAR T-cell therapy, but there still needs to be that note of caution.
Dr. Phillips: Thankfully, we know that limited use of bispecifics doesn’t appear to impact the efficacy of CAR T-cell therapy. We’ve had several data sets that show that you can still successfully harvest and administer CAR T-cell therapy in patients who’ve been exposed to bispecifics. That was a big concern that everyone had. But so far, based on a French and a US study, it doesn’t seem like that is much of an issue in that patient population.
Because they’re off the shelf, the accessibility of bispecifics is there. As these drugs move into other disease settings, such as solid tumors, you’ll likely see wider adoption and more incentive for community sites to use them because they won’t be doing them for the limited number of lymphoma patients. If they’re giving it for lung cancer, breast cancer, and prostate cancer, there are far more patients in that sense. Financially, it gives them more of a reason to have a structure in place for bispecifics and also the medication they need in case things go wrong.
Dr. Evens: There are two off-the-shelf CAR T-cell therapies in clinical trials, which are the so-called allo-CARs. The current agents we have FDA-approved are good, but there are still barriers and we need them to transcend more throughout the community for our cancer treatment.
Stephanie: For clarification, Dr. Phillips, there was some concern about bispecifics impacting the efficacy of CAR T-cell therapy given after, but that hasn’t proved to be the case. You were talking about bridging as well. What I’m hearing is it’s looking promising in terms of the optionality that is here at the fingertips and in research. Is that right?
Dr. Phillips: At this point, especially for the patients who Dr. Evens has talked about and those who maybe can’t get to CAR T-cell therapy for whatever reason, at least with these, we can maybe stabilize the disease enough to allow them the opportunity to get the CAR T-cell therapy if it’s feasible. In some patients, they won’t be able to get to CAR T-cell therapy because they need a lot more support than other treatments so that all has to be in place.
Latest Treatments in Follicular Lymphoma
Stephanie: Follicular lymphoma is very different in that it’s a slow-growing, indolent disease. Let’s start with the relapsed/refractory follicular lymphoma patient population. Can we set the stage by going over what’s available right now and some of the updates?
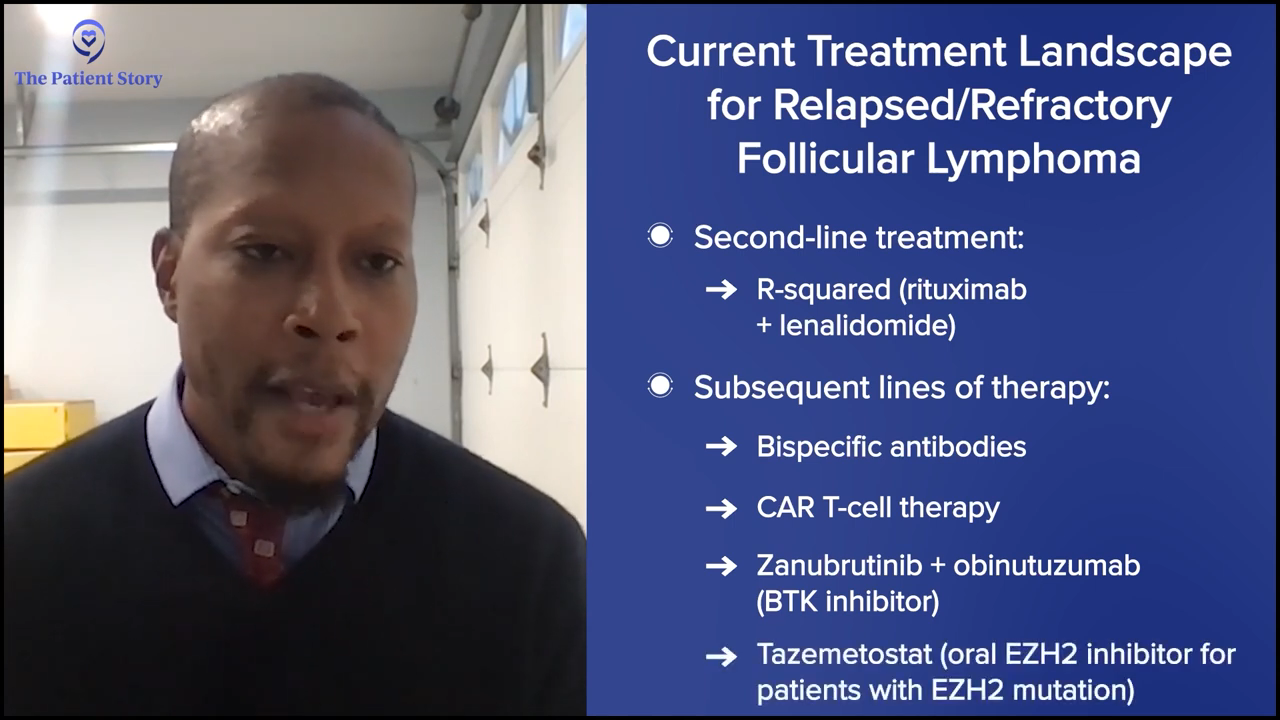
Dr. Phillips: In the relapsed/refractory phase, most people will use R2 (Revlimid and rituximab) based on the AUGMENT study, which showed a significant improvement over rituximab in this patient population. A lot of us are very comfortable with the use of that medication, and the efficacy and tolerability seem to justify it in the second-line space.
After that, it gets a little bit more variable. You have bispecific antibodies approved: mosunetuzumab and epcoritamab. CAR T-cell therapy is available in follicular lymphoma, albeit a little bit of a different argument. CAR T-cell therapy doesn’t necessarily have that curative label as it does in large cell lymphoma, so there’s a lot more variability about what you use in that space.
Recently, we had zanubrutinib and obinutuzumab based on the ROSEWOOD study, which is the first time a BTK inhibitor has shown any benefit for follicular lymphoma. Then there’s tazemetostat, which is an oral EZH2 inhibitor for patients with an EZH2 mutation or those who are deemed to be unfit for other agents, which are all approved in the relapsed/refractory follicular lymphoma space.
Top Developments in Relapsed/Refractory Follicular Lymphoma
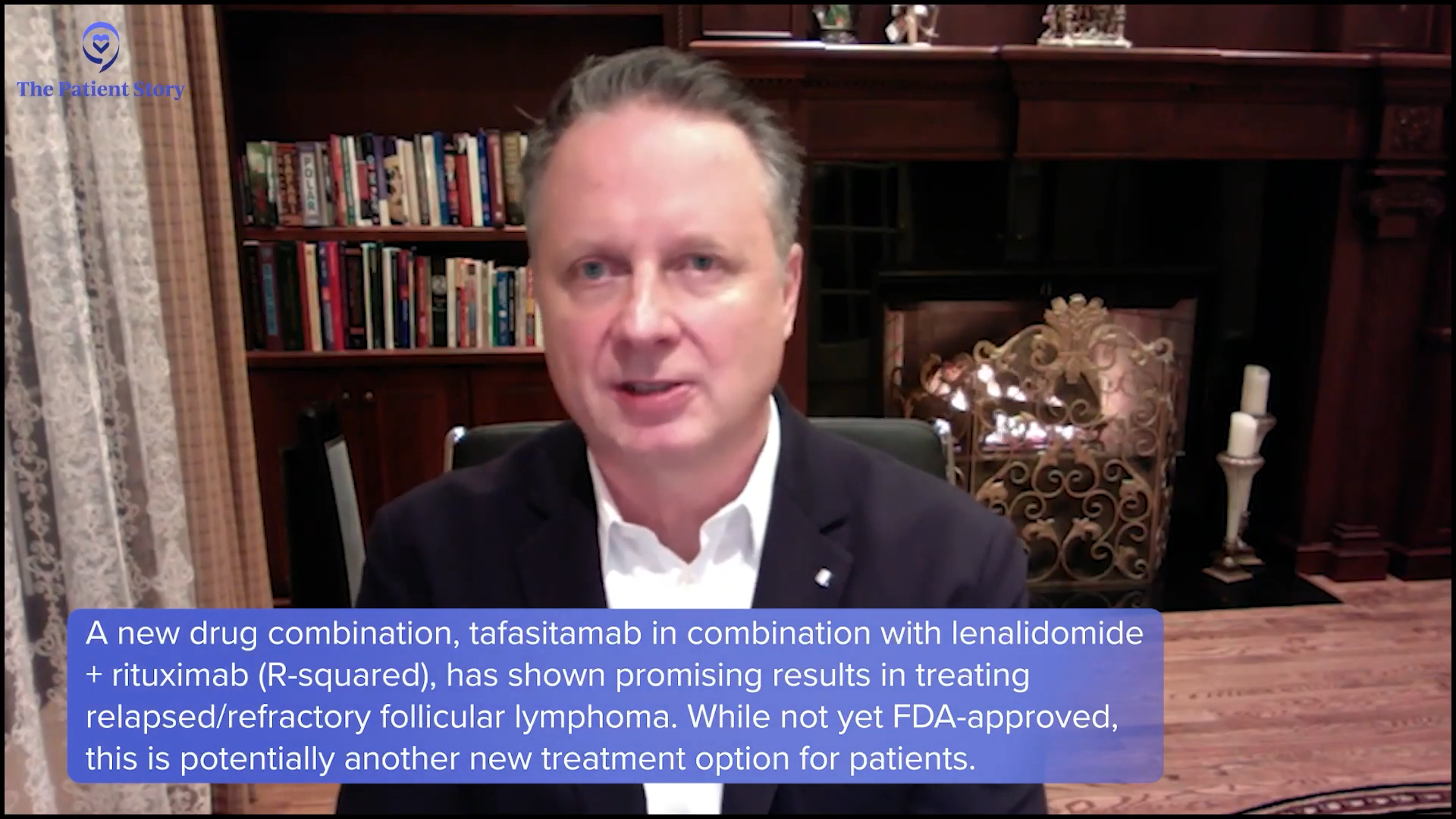
Stephanie: Dr. Evens, there was a late-breaking abstract at the 2024 ASH meeting from the phase 3 InMIND study, which looked at tafasitamab plus lenalidomide and rituximab for the relapsed/refractory situation. Can you provide highlights from this and why patients should pay attention?
Dr. Evens: This was an important presentation and abstract, which was presented by Dr. Laurie Sehn from BC Cancer. It was built off the R2, Revlimid (lenalidomide) in combination with rituximab, which is commonly used in the second-line setting. It was a placebo-controlled study of R2 plus or minus tafasitamab, a CD19 antibody that is already FDA-approved for relapsed/refractory diffuse large B-cell and looking at its entry into follicular lymphoma. It was relapsed/refractory follicular lymphoma, so you had to at least have received rituximab therapy, second line, some somewhere beyond.
It was very effective. There are certain things we look at called hazard ratios and percentage points. Relatively speaking, there was a 57% improvement in progression-free survival, which was pretty significant. If you want to look at it by absolute months, it was on the median of about 12 months, a little bit less than 12 months benefit. This is a presentation. It doesn’t mean it’s FDA-approved although it’s pretty encouraging. I would hope and expect that soon, it will be submitted and eventually be an FDA-approved option for patients.
Dr. Phillips: I was surprised when I saw it and even more surprised about the results.
Dr. Evens: I like tafasitamab. There are no side effects and sometimes it works, but obviously, it’s a tougher patient population than diffuse large B-cell lymphoma. But it had very impressive efficacy and tolerability. We’ve known through the diffuse large B-cell approval that it’s a pretty tolerable medication.
Dr. Phillips: One key point also is that the standard of care arm performed worse than the AUGMENT study, but they enrolled patients with worse disease burden than AUGMENT did. It was a harder-to-treat patient population and they still had very good results that look better than what we got on AUGMENT, so hats off to them because they addressed the elephant in the room ahead of time and had data to support it.
How Should Patients Interpret the Results?
Stephanie: Compared to what’s approved for relapsed/refractory follicular lymphoma, Dr. Evens, how would you explain to patients one side’s already approved and the other side we’re talking about is promising data in research?
Dr. Evens: Like many clinical trials, it’s more than encouraging. It was a phase 3 head-to-head study and you’re not sure of the comparison. It’s a little bit of a waiting game. Sometimes what happens in the United States is there’s a panel called the National Comprehensive Cancer Network (NCCN) that will sometimes list it on its site before FDA approval, although usually they like to see a peer-reviewed manuscript and FDA approval. It emboldens the point of how important clinical trials are. For all of non-Hodgkin’s lymphoma and Hodgkin’s lymphoma for that matter, there are so many new and exciting treatments, but they have to be done with caution on a controlled clinical trial.
Bispecifics and Follicular Lymphoma
Stephanie: We talked extensively about bispecifics in DLBCL. What has the response been in follicular lymphoma and relapsed/refractory follicular lymphoma?
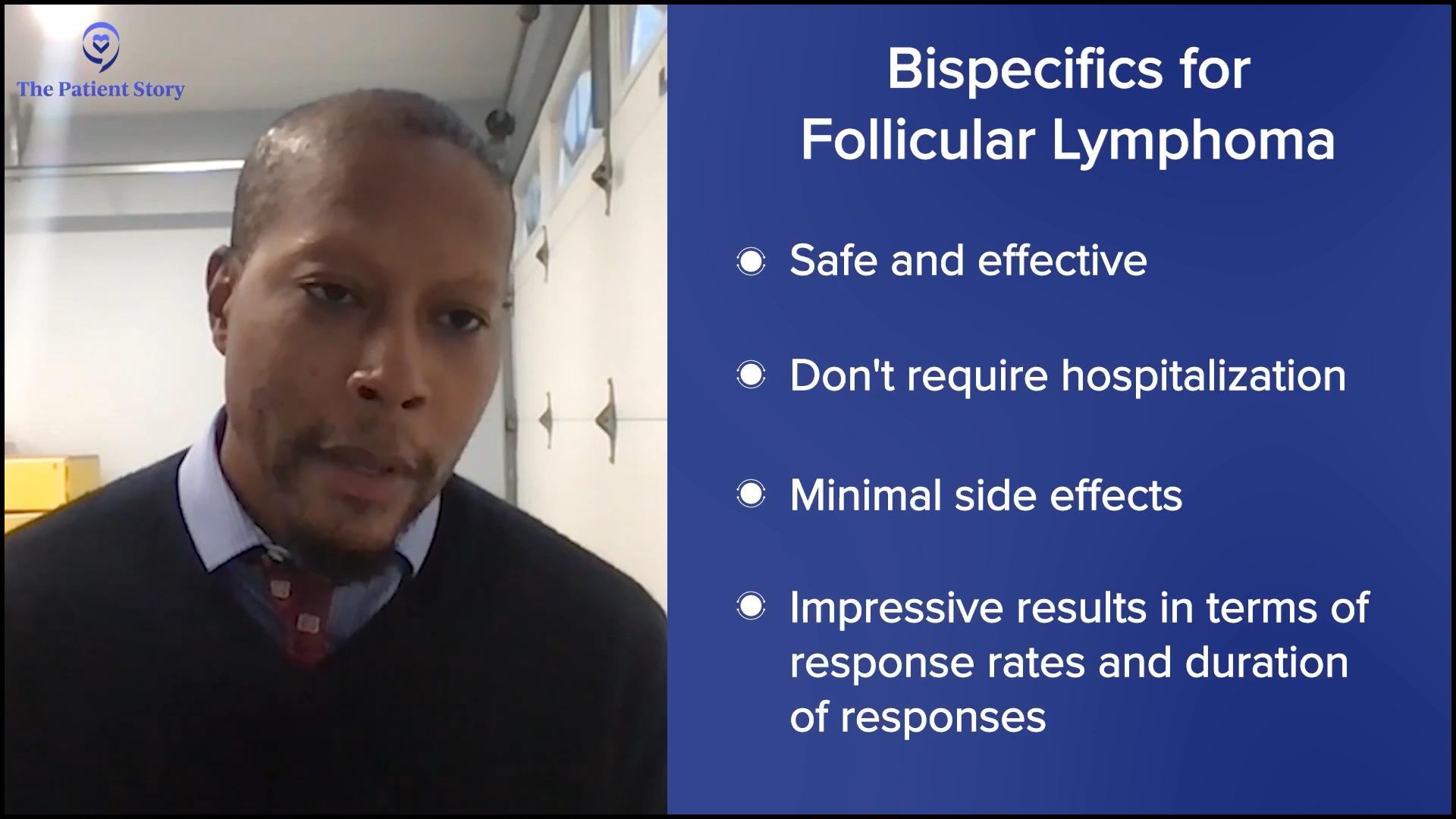
Dr. Phillips: It’s been good. The data has been very impressive. They have longer follow-up mosunetuzumab compared to epcoritamab, but that’s because it was approved sooner. I would think most people would prefer a bispecific over CAR T-cell therapy in a very similar patient.
There are some caveats. Some patients have more aggressive disease and more concerning features where you would probably go to CAR T-cell therapy. The safety of these drugs and lack of hospitalization are important. None of the ones approved in follicular lymphoma require patients to be hospitalized for observation and have very low rates of CRS. Mostly the ones that we see are grade 1, very few grade 2, and again, high overall response rate, high complete response rate, and very impressive durability of response.
Pending how things appear when the patient relapses, like if they still express CD20, it does offer the opportunity to retreat these patients with a bispecific, which is some of the stuff that future research is looking to determine whether they can still get very durable responses on second treatment. There have been very small data sets so far about the retreatment aspect, but that’s the opportunity that bispecifics can offer patients, even in a community setting without having to travel far from home.
Dr. Evens: Sometimes these diseases, even follicular lymphoma or other indolent lymphomas, can pick up ahead of steam and cause symptoms and that usually is where it gets large or becomes a trigger to start treatment. It’s treatable. We know now through randomized trials that life is extended with many of these therapies.
Generally speaking, it’s still not curable. We’re going to keep doing clinical trials and shooting for that cure. Not that quality of life is not important in diffuse large B-cell, but it’s ultra-important in follicular lymphoma. Hopefully, most if not all of these trials have some embedded quality-of-life measures to make sure that not only is the treatment effective, but it also allows patients to maintain their quality of life.
Over a 20-or 30-year period, some patients with follicular lymphoma may go through six, seven, or eight different treatments. Our hope is during that length of life, a good chunk of it is off-treatment and that’s why so many of these studies, thankfully, are being done with time-limited treatment. You treat for six months, a year, or even 18 months, pause, and hopefully patients can enjoy that remission off-treatment for as long as possible.
Dr. Phillips: He summarized the dilemma in follicular. You’re less willing to take toxicity because longevity and quality of life are paramount.
Dr. Evens: We presented a poster at ASH. Dr. Vaishalee Kenkre at the University of Wisconsin was the lead author. We have the Big Ten Cancer Research Consortium where we’re able to study. A study that Dr. Kenkre brought up is a common treatment, bendamustine-rituximab, in the front-line setting, given usually for six months. She brought tazemetostat, one of the medications Dr. Phillips mentioned, to the front-line setting and only gave three cycles of bendamustine-rituximab. There was a very early, response rate of 100% so far. But again, every trial in many ways is a good clinical trial. Hopefully, we can shift many of these novel and targeted treatments to the frontline that are more tolerable and time-limited, and garner long remissions without cytotoxic chemotherapy.
Dr. Phillips: Tazemetostat is one of the more tolerable oral medications I’ve ever seen.
Dr. Evens: It is and it’s remarkable. It’s very early. It was only 12 or 13 patients, but it’s a lead-in study, so you get tazemetostat and then concurrent taz-BR. It’s agnostic of the EZH2 mutation. We’ll have to see how that pans out, whether it matters or not in the front-line setting.
Dr. Phillips: The big point you said is no added toxicity, which we saw with a lot of other drugs. Lenalidomide is toxic with bendamustine. Even BTK inhibitors can cause some problems with bendamustine.
Dr. Evens: That’s right. It was investigator-initiated and thankfully, we were able to convince the company not to add it on to six of bendamustine-rituximab, but let’s try and do a short course of bendamustine. We’ll see how that pans out. Almost all of the medications in some form or fashion are being looked at in clinical trials in the front-line setting and that’s what we want — super-targeted, effective, incredibly well-tolerated, yet time-limited therapy for follicular lymphoma and other indolent lymphomas.

Key Takeaways
Stephanie: We’ve talked a lot about the research that’s happening and what’s already out there. What would you say to patients and their care partners in terms of the landscape over the next few years, both in DLBCL and in follicular? What are the trends and what are you excited by for patients and care partners?
Dr. Phillips: In large cell lymphoma, we are moving towards more personalized care. Even though we’re adding extra medications, it doesn’t necessarily mean we’re adding extra toxicity, which hopefully gives more efficacies without impacting quality of life or even adding long-term side effects to the patient profile. It’s all good news for the patients because everything for the most part is a specifically targeted treatment and not necessarily as generalized and causing a lot of side effects.
I’m excited to see where front-line large-cell lymphoma goes. Even in the relapsed/refractory space, as Dr. Evens alluded to, there are more and more treatments coming along. We are incrementally increasing the number of patients we’re curing from this cancer. The fewer patients there are in the relapsed/refractory space, the fewer patients we lose to this cancer, which is important and a big leap. Five years ago, you could routinely say that about 35% of patients would probably have very bad outcomes and there wasn’t much we could do about it.
Follicular lymphoma has become like what we see with Hodgkin’s without the ability to cure, but we’re trying to improve what we’re doing for patients with a very high recognition of tolerability. Anything moving up and being added are drugs that are safe and effective. Safety is paramount, so the quality of life that patients deserve and the extended survival will be time that they can enjoy with their family without worrying about side effects that come from these treatments.
We’ve already talked about tafasitamab, lenalidomide, and rituximab. We’ll have the bispecifics. Tazemetostat is being explored with R2. Eventually, some of these things may move into the front-line space and we can re-challenge the role of where chemotherapy fits in our patient population, even with time-limited therapies without cytotoxic drugs. It’s a very exciting time as we move forward. It may be more confusing for us as physicians because we’ll have more options, but that usually always equals good outcomes for patients.

Dr. Evens: Tycel did an amazing job of giving a summary, so I’m going to put a pin in a word he said: exciting. It’s an exciting time for investigators, like Dr. Phillips and myself, but also for patients and caregivers. There are a multitude of novel, targeted treatment options. We talked a lot about immunotherapy but also even some oral, targeted therapies that are on our shelves now as we speak and probably 100-plus new ones coming down the pipeline. What does that mean for patients? It hopefully means a lot of good things and a lot of more effective and tolerated therapy.
For patients and caregivers, always ask questions. Please never be hesitant to ask your oncologist a question. What are all of my treatment options, whether on the shelf or on a clinical trial? Never hesitate to bring up a side effect you have, whether it’s numbness, tingling, fever, or something more significant. Open lines of communication are paramount.
Patients have their family members and doctors, but there are so many good societies and foundations out there that can be a great anchor for patients to talk to other patients and get information. There’s a lot of great information in a fast-moving field but in all great directions.

Conclusion
Stephanie: Thank you so much, Drs. Evens and Phillips, for being here, for the work that you do, and for going way above and beyond treating your patients. We look forward to featuring you again soon.
With that, we wrap up another wonderful discussion at The Patient Story. I appreciate these doctors who take time out of their busy schedules to provide great insights for us in a way that we can understand better. It’s so important to be empowered, to lean into this communication, and to have more conversations about what’s impacting your quality of life so that you as patients, caregivers, and care partners can work together with the medical teams to stay on treatments that are effective and live life the way that you want to.
Thank you again to our partner, The Leukemia & Lymphoma Society. Check out the links to the LLS’ different parts of their website for their resources, like the information resource center, which provides free one-to-one support with questions across the board, and their Clinical Trial Support Center, where clinical trial nurses help you find trials, stay on trials, and answer all the questions in between.
We hope that this was helpful to you. Thank you so much for joining and we hope to see you at another program soon. Take good care.

Thank you to The Leukemia & Lymphoma Society for their partnership. The Leukemia & Lymphoma Society is here for you with information about clinical trials, resources, and support.
DLBCL Patient Stories
Ashley P., Diffuse Large B-Cell Lymphoma (DLBCL), Stage 4
Symptoms: Feeling like holding breath when bending down or picking up objects from the floor, waking abruptly at night feeling “off,” one episode of fainting (syncope), presence of a large mass in the breast
Treatments: Chemotherapy, bridge therapy of chemotherapy and radiation, CAR T-cell therapy
Melissa B., Relapsed Diffuse Large B-Cell Lymphoma (DLBCL)
Symptoms: Lump in the left breast, persistent rash (started near the belly button and spread), intense fatigue and energy loss
Treatments: Chemotherapy (R-EPOCH), Neulasta, radiation therapy, surgery (to remove scar tissue and necrosis), autologous stem cell transplant
Jen N., Diffuse Large B-Cell Lymphoma (DLBCL), Stage 4B
Symptoms: Blood-tinged phlegm, whole-body itching, shortness of breath, lump near collarbone, night sweats, upper body swelling, rapid weight loss
Treatments: Chemotherapy, immunotherapy, lumbar puncture, autologous stem cell transplant
Jim Z., Diffuse Large B-Cell Lymphoma (DLBCL)
Symptoms: Sudden and severe head and neck swelling, purplish facial discoloration, bulging neck veins
Treatments: Surgery (resection and reconstruction of the superior vena cava), chemotherapy
Follicular Lymphoma Patient Stories
Hayley H., Follicular Lymphoma, Stage 3B
Symptoms: Intermittent feeling of pressure above clavicle, appearance of lumps on the neck, mild wheeze when breathing and seated in a certain position
Treatments: Surgery, chemotherapy
Laurie A., Follicular Non-Hodgkin Lymphoma, Stage 4
Symptoms: Frequent sinus infections, dry right eye, fatigue, lump in abdomen
Treatments: Chemotherapy, targeted therapy, radioimmunotherapy
Courtney L., Follicular Lymphoma, Stage 3B
Symptoms: Intermittent back pain, sinus issues, hearing loss, swollen lymph node in neck, difficulty breathing
Treatment: Chemotherapy
John S., Follicular Lymphoma, Stage 4
Symptom: Swollen lymph nodes
Treatments: Clinical trial, chemotherapy