Cancer Clinical Trials
The Latest in Myelofibrosis Treatments
Edited by:
Katrina Villareal
Interested in myelofibrosis clinical trials? We’ve gathered the leading experts to explain emerging clinical trials, misconceptions, and how to find the treatment best for you.
Moderated by The Patient Story founder Stephanie Chuang and myelofibrosis advocate Ruth Fein Revell, this discussion features Dr. Angela Fleischman (UC Irvine Health), Dr. Ruben Mesa (Atrium Health Wake Forest Baptist), and clinical trial nurse Melissa Melendez (The Leukemia & Lymphoma Society).
Gain invaluable insights into upcoming exciting clinical trials, the purpose and benefit of trials, and navigating trials and treatments. Optimize communication with your healthcare team and empower yourself with extra support and resources.

Brought to you in partnership with The Leukemia & Lymphoma Society

Thank you to GSK for its support of our patient education program! The Patient Story retains full editorial control over all content.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider for treatment decisions.
- Introduction
- Ruth’s MPN Story
- What is Myelofibrosis?
- Advances in Myelofibrosis Treatments
- Standard of Care: First-Line Treatment
- How JAK Inhibitors Work
- Navigating Treatment Options
- Combination Therapy Clinical Trials
- Trials Beyond JAK Inhibitors
- Treatment for Patients Who Suffer From Anemia
- Symptom Control While Being on Treatment
- The Best Time to Get a Stem Cell Transplant
- Asking About Clinical Trials
- Trial Knowledge #1: Phases of Clinical Trials
- Trial Knowledge #2: Use of Placebos in Clinical Trials
- Trial Knowledge #3: Finding Clinical Trials
- Trial Knowledge #4: Staying in a Clinical Trial
- Final Takeaways
Introduction
Stephanie Chuang, The Patient Story: Hi, everyone! This discussion is hosted by The Leukemia & Lymphoma Society and The Patient Story.
I am the founder of The Patient Story and I also got a blood cancer diagnosis. Mine was in non-Hodgkin lymphoma but there are so many shared experiences in what we’re all looking for and that is what The Patient Story is all about.

We try to help patients and their supporters navigate a diagnosis and we do this through in-depth conversations with patients, care partners, and top specialists across different cancers.
It’s important to have these educational programs, especially when it comes to topics like clinical trials, which are daunting and overwhelming, so we’re trying to humanize your options.
We’re so proud to be co-hosting this with The Leukemia & Lymphoma Society, the world’s largest non-profit health organization dedicated to funding blood cancer research as well as offering patient services and education.
They have great resources, including information specialists who are a phone call away to help answer your questions. We’ll be highlighting the Clinical Trial Support Center, a very critical free resource that offers one-on-one support to enroll in and stay in clinical trials.
We also want to say special thanks to GSK for supporting our educational program, which helps us make this available and free for our audience. The Leukemia & Lymphoma Society and The Patient Story retain full editorial control of the entire program.
This is not meant to be a substitute for medical advice. We want you to walk away and be able to ask your own doctors and medical teams questions about clinical trials in myelofibrosis.
I know how fortunate I am as many people suffer so much more than I ever have.
Ruth Fein Revell
Ruth Fein Revell, Patient Advocate
Stephanie: I’m thrilled to introduce someone who’s not only a big MPN and myelofibrosis patient voice and leader in the community but also someone I’m lucky to call a friend and who will be leading the conversation.
Ruth, I know that you’re going to share more about your own myelofibrosis cancer story and how you became a passionate advocate. But first, can you share more about yourself outside the cancer context? Because as we know, we are so much more than a diagnosis.
Ruth Fein Revell: Thanks so much, Stephanie. Professionally, I’m a health and science writer who now writes primarily about cancers, blood cancers in particular. I also host patient programs. I have the privilege of speaking to many of the world’s most prominent researchers and clinicians.
In many ways, I think cancer has attracted many of us because we feel we can make a real difference in a difficult disease.
Dr. Ruben Mesa
Dr. Ruben Mesa, Hematologist-Oncologist
Ruth: Dr. Ruben Mesa is a renowned hematologist-oncologist. He’s currently the president of Atrium Health Levine Cancer Institute and senior vice president of Atrium Health. He’s also the executive director of the NCI-designated Wake Forest Baptist Comprehensive Cancer Center and the vice dean for cancer programs at Wake Forest School of Medicine.

He’s globally recognized as a leader in the MPN space and we are so fortunate to have him with us. Our audience would love to know you beyond that white coat of yours. What drew you to work in cancer and research?
Dr. Ruben Mesa: Cancer is such a terrible disease. It impacts and steals from an individual’s length of life and quality of life. I myself have been touched by cancer as so many have. My father passed from cancer and my mother is a cancer survivor. I was able to witness the impact of cancer research, developing new therapies, and the value of really compassionate care and how important these things were.
In many ways, I think cancer has attracted many of us because we feel we can make a real difference in a difficult disease. It’s also an exciting time when scientific progress is really making a genuine impact.
There are many aspects of myeloproliferative neoplasm that I find extremely rewarding to take part in.
Dr. Angela Fleischman

Dr. Angela Fleischman, Hematologist-Oncologist
Ruth: Dr. Angela Fleischman is a hematologist-oncologist at UC Irvine Health, where she leads the Fleishman Lab with a special focus on MPNs and improving the options and care for patients.
As a physician-scientist, Dr. Fleischman not only treats patients but also actively researches hematological malignancies. She’s passionate about translating findings from the lab bench to the patient’s bedside.
Dr. Fleischman, we’d love to know a little more about your passions as well. What drew you to blood cancers and research?
Dr. Angela Fleischman: I have always been extremely interested in blood cell development. I started out as a Ph.D. student prior to my MD and focused on normal blood cell development and what determines whether a blood stem cell goes one direction or the other.
When I decided to go to medical school and first learned about myeloproliferative neoplasms, I was extremely fascinated by them because I felt that it was an opportunity to learn what happens when blood cell development goes awry.
Another extremely interesting aspect of myeloproliferative neoplasm that I was extremely drawn to is the ability to make connections with patients throughout the years because it’s a chronic disease. As a physician, I’m able to travel with them through their journey as a patient. There are many aspects of myeloproliferative neoplasm that I find extremely rewarding to take part in.
I help patients navigate the complicated clinical trial landscape, and provide them with education and potential treatment options to take back to their care team.
Melissa Melendez
Melissa Melendez, CTSC Nurse Navigator
Ruth: Melissa Melendez is a nurse navigator with The Leukemia & Lymphoma Society’s Clinical Trial Support Center. Melissa, what drew you to become a nurse and to the important work that you do?
Melissa Melendez: I’ve been in the oncology field for almost 20 years and it was a happy accident how it happened. While in nursing school, I started my first clinical rotation in the oncology unit. I was pretty apprehensive and concerned that I was being plunged into caring for such complex patients with extreme highs and extreme lows of treatment outcomes.
I instantly fell in love with not only helping patients on their journeys throughout their treatment but also the privilege of caring for their families who were going through the journey as well. I decided that oncology was going to be my career path.
I started as a patient care assistant then a registered nurse, a division supervisor, and currently a nurse practitioner. Working for The Leukemia & Lymphoma Society in the Clinical Trial Support Center has been such a blessing for me as a nurse and it was a pretty easy transition from my previous occupation.

I help patients with clinical trials and throughout their journey. Not only do I work with intelligent, caring, and selfless colleagues but also with a population of patients and families that I truly love.
On a daily basis, I get to see the impact that the LLS has on patients and families, help patients navigate the complicated clinical trial landscape, and provide them with education and potential treatment options to take back to their care team to discuss so they can make informed decisions about their care. I get to build relationships with patients and family members that last for years.
It wasn’t until I was taken off hydroxyurea before starting a clinical trial that I realized how severe my symptoms were without treatment.
Ruth Fein Revell
Ruth’s MPN Story
Ruth: What is myelofibrosis? Myelofibrosis is one of the MPNs, a myeloproliferative neoplasm, and a very personal topic for me. I’ve lived with an MPN for nearly 30 years, originally diagnosed with essential thrombocythemia or ET at age 38 while I was raising two young boys.
I was taking an aspirin a day, which was stopped because I developed an ulcer. I ended up with clots in my portal and splenic veins. After a week in the hospital, I was put on an anticoagulant or a blood thinner. Several years later, my bone marrow flipped a proverbial switch and instead of producing too many platelets, it now produced too many red blood cells.

The diagnosis was changed to polycythemia vera and I lived with periodic phlebotomies and daily hydroxyurea. That was part of my life, as with many people who live with polycythemia vera and the different MPNs.
In 2018, I had surgery for early colon cancer. The surgeon was so focused on avoiding any more clots that I had the opposite happen. A major bleeding episode put me in the ICU for nearly a week, followed by several months of healing.
I finally sought out an MPN specialist and began to see Dr. Ellen Ritchie at Weill Cornell in New York. I had an updated bone marrow biopsy, which confirmed a diagnosis of myelofibrosis.
I used to say I was mostly asymptomatic but I’ve lived with severe migraines, a few TIAs (transient ischemic attacks), and other constitutional symptoms my whole life. It wasn’t until I was taken off hydroxyurea before starting a clinical trial that I realized how severe my symptoms were without treatment.
I experienced both life-threatening clots and bleeding episodes. I had such extreme fatigue that I couldn’t walk up the street without stopping to lean or sit on a neighbor’s step. Of course, I also know how fortunate I am as many people suffer so much more than I ever have.
Fibrosis does not always equal the MPN myelofibrosis.
Dr. Angela Fleischman
What is Myelofibrosis?
Ruth: Let’s dig right in. Dr. Fleischman, what exactly is myelofibrosis?
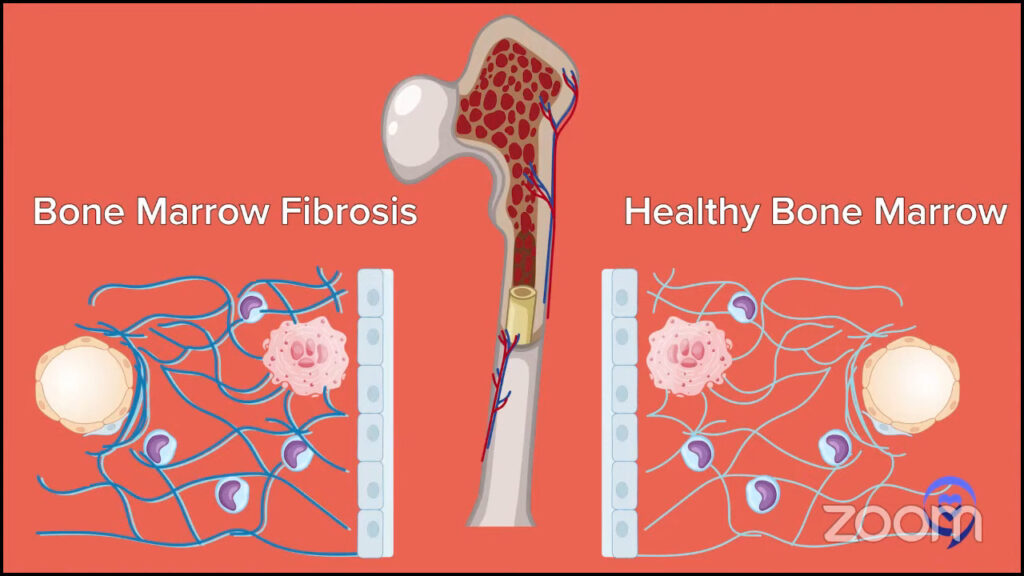
Dr. Fleischman: People may interpret the word myelofibrosis as having some fibrosis in the bone marrow. What we’re talking about is the myeloproliferative neoplasm myelofibrosis, which also can have some fibrosis in the bone marrow. However, I want to emphasize that fibrosis in the bone marrow does not always equal myelofibrosis.
MPN myelofibrosis is a chronic blood cancer that stems from the proliferation of some abnormal mutant cells in the bone marrow. Exactly what causes the fibrosis is unclear. The abnormal megakaryocytes or the cells that are producing platelets have been implicated as the bad cells in myelofibrosis and causing the fibrosis.
Patients also tend to have an enlarged spleen, which can also be seen in ET and PV but is more prevalent in myelofibrosis.
Blood counts can look very different. There’s a wide variety of what a myelofibrosis patient’s blood counts look like, but a classic myelofibrosis patient may have some anemia. They may have a little bit of a high white blood cell count. They may also have immature cells in their blood so their bone marrow cells are not maturing fully before going into the blood, indicating that there’s some stress going on in the bone marrow.
Myelofibrosis patients can have some significant constitutional symptoms. ET and PV can also, but in myelofibrosis, they tend to be more severe.
I do want to emphasize that because somebody has a bone marrow biopsy that says fibrosis does not necessarily always equal the MPN myelofibrosis. Other things, like autoimmune diseases, can cause some fibrosis in the marrow. Infections can cause fibrosis in the marrow. Other blood cancers can cause fibrosis in the marrow. What I want to emphasize is fibrosis does not always equal the MPN myelofibrosis.
Ruth: That’s a really good reminder and not something that people focus on much so thank you for that.
We’ve seen an explosion in many different types of therapies. Over the last three years, we’ve seen a rapid increase in FDA-approved options.
Dr. Ruben Mesa
Advances in Myelofibrosis Treatments
Ruth: The world of MPNs, in particular myelofibrosis, is changing before our eyes as clinicians, researchers, and people living with these conditions. Dr. Mesa, would you tell us how the landscape of treatments for MF patients has changed so dramatically in the last 2 to 3 years?
Dr. Mesa: I first saw patients with myelofibrosis at the beginning of my training almost 30 years ago. In the first half of those 30 years, the medicines we had for myelofibrosis were really limited because we had a very limited understanding of the biology of the disease.
What happened about 15 years ago is we identified important genetic changes, such as JAK2, CALR (calreticulin), MPL, and others that lead downstream to a patient having a myeloproliferative neoplasm.

With that, we’ve seen an explosion in many different types of therapies. Over the last three years, we’ve seen a rapid increase in FDA-approved options, now four different JAK2 inhibitors. They’ve made a real impact in patients with myelofibrosis regarding the enlargement of the spleen, perhaps helping to slow down the course of the disease to some degree and, for some individuals, dramatically so.
The next step we’re launching into is combinations of therapies where we use more than one drug and look at treating different aspects of the disease or different targets in terms of the biology of the disease to make a deeper impact.
Wearing my cancer center hat, as I look at how therapies are advancing in many different diseases, both blood cancers as well as non-blood cancers, most of the time we’re now using more than one therapy where medicines complement each other.
Different blood diseases, such as multiple myeloma, use up to four different drugs at a time. The explosion went from really very little understanding to multiple drugs, a much greater understanding of the biology of the disease, and hopefully a deeper and deeper impact.
For the majority of individuals, probably over 90%, based on a variety of factors, we start with medicines.
Dr. Ruben Mesa
Standard of Care: First-Line Treatment
Ruth: Dr. Mesa, what is the standard first-line treatment for myelofibrosis?
Dr. Mesa: When patients come in with a diagnosis of myelofibrosis, their doctor first gets a sense of the risk of the disease, how likely is the disease to be life-threatening, and the burden that the patient is facing from an enlarged spleen, from symptoms, or from the risk of progression.
We discuss whether we should pursue the potentially curative option of bone marrow transplant or stem cell transplant; we use those terms interchangeably. That’s a very complex therapy, but it’s something that we consider throughout for individuals up until their 70s, depending on the risk of the disease and other factors.
For the majority of individuals, probably over 90%, based on a variety of factors, we start with medicines. The only approved medicines for myelofibrosis are the JAK inhibitors. Transplant either in the near future, delayed, or we’re not going to pursue transplant and then consideration of a JAK inhibitor.

JAK inhibitors help to decrease the activation of JAK2. Regardless of which mutation a patient has or even if they don’t have one of the three mutations, they all seem to benefit from JAK inhibitors.
Dr. Ruben Mesa
How JAK Inhibitors Work
Ruth: We currently have four FDA-approved JAK inhibitors, including momelotinib, which was approved in September 2023. How do JAK inhibitors work? For each of the approved JAK inhibitors, would you explain what their benefits and limitations are?
Dr. Mesa: In myelofibrosis, there are three main mutations that we believe are involved with causing the disease. The JAK2 or the JAK2 V617F, the mutation in calreticulin, and the mutation in MPL. There’s even a small subset of people who do not have one of those three.

All of those mutations lead to the same outcome: an overactivation of the protein JAK2. JAK2 is one step. It’s an on-off switch stuck in the on position telling cells to grow.
JAK inhibitors help to decrease the activation of JAK2. Regardless of which of those mutations a patient has or even if they don’t have one of those three mutations, they all seem to benefit from JAK inhibitors.
Ruxolitinib has been approved since 2011. It improves the spleen and symptoms, may help patients who respond live longer with the disease, and in many ways has long been the standard. It’s frequently been used as the base in combination trials.
Fedratinib has been approved since 2019. It can be used to improve the spleen or symptoms. It may be used in the front-line setting instead of ruxolitinib or in the second-line setting if someone has had ruxolitinib already.
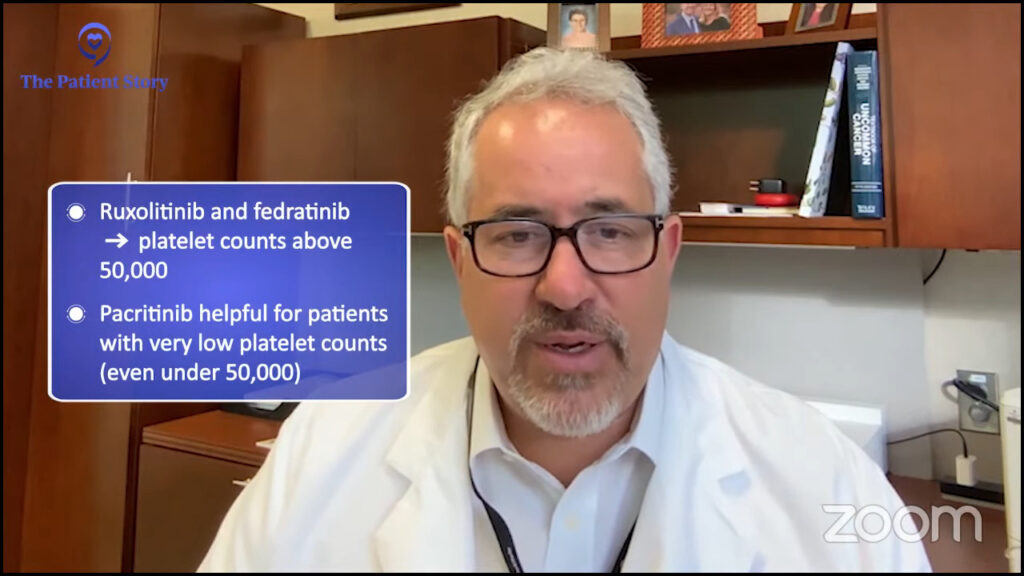
Pacritinib was approved in 2022 and this one has a little distinct profile. It’s particularly helpful for decreasing the spleen and symptoms but for individuals with a very low platelet count.
Both ruxolitinib and fedratinib are approved for individuals with a platelet count above 50,000. Pacritinib can be helpful in anyone, but its particularly unique niche is in individuals under 50,000 and we certainly will consider it for individuals with lower blood counts.
Momelotinib was approved in September 2023. This can improve the spleen symptoms and anemia. It showed the strongest improvement in anemia compared to the other three. Pacritinib sometimes can have an improvement in anemia. Unfortunately, both fedratinib and ruxolitinib sometimes can have anemia as a side effect or worsen anemia and rarely would improve anemia.
There are some differences among the four of them, but they overlap in many ways in terms of improving the spleen and symptoms and can be beneficial regardless of the mutation profile that a patient with myelofibrosis has.
Ruth: It’s very exciting for the MPN community to finally have so many treatment options. People often think that because they have mutations other than JAK2, a JAK inhibitor won’t help them so thanks for noting that that’s not necessarily the case.
It’s really exciting now that we have four different JAK inhibitors so we can try to tailor the best JAK inhibitor for the person upfront.
Dr. Angela Fleischman
Navigating Treatment Options
Ruth: Dr. Fleischman, here’s a loaded question. How do you know which JAK inhibitor may or may not work and which one to try first?
Dr. Fleischman: This is an evolving topic. It’s really exciting now that we have four different JAK inhibitors so we can try to tailor the best JAK inhibitor for the person upfront. Sometimes it’s a hit or miss but, as Dr. Mesa described, each JAK inhibitor has a little bit of a different profile.

All of the JAK inhibitors inhibit JAK2 but each one has “off-target” effects and some different proteins and that may explain why they have their own unique profile.
Each myelofibrosis patient is quite unique so they have different needs. For example, for somebody who has an enlarged spleen, with symptoms, and pretty robust blood counts, ruxolitinib would be a great option, which is also the only JAK inhibitor currently approved for PV, in which the purpose is to bring down blood counts.
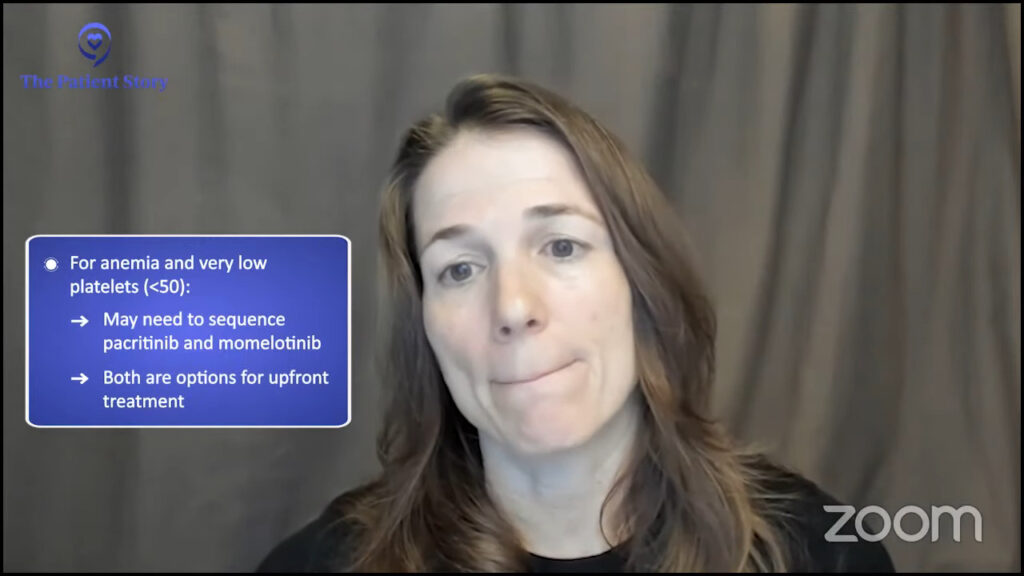
If the person’s primary problem is anemia, which is unfortunately a very common problem in myelofibrosis, it’s very exciting that we have an option that’s specifically designed for patients with anemia. That’s a patient population very, very appropriate to start pacritinib first.
Many patients with anemia also have a low platelet count. What do you do for somebody who has both anemia and a very low platelet count? We’re going to have to learn how to sequence and make decisions between pacritinib and momelotinib. For a myelofibrosis patient with both anemia and thrombocytopenia or low platelet count, the two options that exist upfront are momelotinib and pacritinib.
Different tiers of needs as we talk about individual clinical trials.
Dr. Ruben Mesa
Combination Therapy Clinical Trials
Ruth: Some of the most exciting clinical trials for MF patients are for combination therapies. Dr. Mesa, can you explain the idea behind combination drug approaches and who might benefit?
Dr. Mesa: We think about different populations of patients. We have those who are JAK inhibitor naive or individuals who have not had a JAK inhibitor yet. This may be at the time of diagnosis or individuals who have had the disease for a while, have been observed, and now, based on spleen symptoms or changes, need to begin therapy.

There are individuals who have been on a JAK inhibitor and have had some benefit, but we feel that there’s an opportunity for further benefit. Some of these individuals are participating in add-on studies where you have a JAK inhibitor that they’ve been on for a while, they have a partial response, and a second drug is added.
The third type of patient is an individual who was on a JAK inhibitor and now no longer has a benefit from that medication so you are switching them to a different therapy altogether. Different tiers of needs as we talk about individual clinical trials.
Selinexor + JAK Inhibitor
Ruth: Let’s talk about some of those combination trials. There are a number of them where JAK inhibitors are given with the addition of a new agent with a different mechanism of action. Let’s talk about selinexor plus JAK inhibitor.
Dr. Mesa: There’s a whole constellation of different agents that have an impact on different mechanisms of action that are being looked at in combination with JAK inhibitors. This one is a little bit earlier in its development.
It’s an approved drug for multiple myeloma and has been approved since July 2019. It’s an anti-cancer medication. It’s a selective inhibitor of nuclear export. It works on some of the processes within the cell in a different way that we feel may be beneficial in combination.

Data from early studies suggest that there is benefit and it may be more with two drugs than the single drug itself. Whether this will become an option for patients with myelofibrosis, the trials will demonstrate, but it has made an important impact for individuals with myeloma so we’re hopeful that it’ll be helpful in this group of patients as well.
Ruth: It’s great to see when we borrow from different areas of research.

Navitoclax + JAK Inhibitor
Dr. Mesa: This works against a process within cells called apoptosis or programmed cell death. Navitoclax is a cousin of a drug that’s approved for acute leukemia called venetoclax.
In studies with myelofibrosis, navitoclax has shown real activity for decreasing spleen symptoms and potentially impacting the disease and, in combination, to a greater degree than JAK inhibitor alone. We have had patients on large phase 3 trials who have been newly diagnosed as well as other situations so those data are anticipated with great interest.
It may take further time for us to identify which combination is a better fit for a patient. These trials are not necessarily designed to answer that itself.
Dr. Ruben Mesa
Pelabresib + JAK Inhibitor
Ruth: Let’s talk about pelabresib, a drug very close to my heart. That’s the trial I’ve been on for more than three and a half years used in combination with a JAK inhibitor. I understand the results have been very promising. At Weill Cornell, they’re calling me the poster child for this study so I’m very grateful for that. Dr. Mesa, tell us about the MANIFEST-2 trial.
Dr. Mesa: This is probably the furthest along of the combination approaches. Pelabresib is an inhibitor of another cellular process called BET. There’s reason to believe that it may have a very complementary role in inhibiting JAK2.
In the early studies, it appeared very promising in terms of having an impact, perhaps more than JAK inhibition alone or in combination when individuals had failed a JAK inhibitor.
We now have phase 3 trials of patients newly diagnosed or who are JAK inhibitor naive receiving this combination versus ruxolitinib alone. We are looking for information to see the net benefit of using the two drugs.
Are the responses more profound? Do they last longer? Are patients better off? We always weigh any downsides to being on two drugs versus one. There are multiple combinations being tested in parallel. It’s possible that multiple of them may be beneficial. It’s possible that multiple of them could become FDA-approved.

It may take further time for us to identify which combination is a better fit for a patient. These trials are not necessarily designed to answer that itself. They’re designed to answer if they are better than a JAK inhibitor alone.
Downstream, we’ll have a better understanding based on specific patient features or other aspects of their health whether one of these combinations may be a better choice for one patient versus the other.
Ruth: Dr. Fleischman, what should patients be asking their doctors to understand if one of these combination therapies might be best for them?

Dr. Fleischman: Entering into a clinical trial is a personal decision for the patient and deserves deep thought. There are many reasons why somebody may want to do a combination therapy. It’s not only because your current therapy isn’t working or that you want something “new” but also to help other people.
Clinical trials are not necessarily only supposed to be for the benefit of the patient themselves, but by participating in a clinical trial, the patient is increasing our knowledge of whether specific combinations work, the appropriate dosing, and the side effects. You’re helping other patients who will come after you.
Cancers are pretty smart that if you target one, they’re going to find a way to get themselves around that medicine you’re giving them.
Dr. Angela Fleischman
Trials Beyond JAK Inhibitors
Ruth: We go into clinical trials often because we were on Jakafi or another treatment and it wasn’t effective, caused side effects that were serious and needed to be discontinued, or maybe it worked for some time and then stopped. Dr. Fleischman, can you talk more about aiming to introduce non-JAK inhibitor treatments?
Dr. Fleischman: JAK inhibitors are very good in terms of reducing inflammation and reducing spleen size and, in some patients, can work very well for a period of time.
Patients could get “resistance.” Resistance with JAK inhibitors is very different than what we think about with other cancers. People on JAK inhibitors don’t develop new mutations that make them “resistant” to the JAK inhibitor. They start to not work as well so that would be one reason to add on and target different pathways.
Myelofibrosis is very heterogeneous. Each patient is very different. Each patient may have different mutations. They may have either JAK2, calreticulin, or MPL, but some patients will have additional mutations that make them look a little bit different than other myelofibrosis patients.

Trying to target multiple pathways, as Dr. Mesa had mentioned, is a very common technique. Cancers are pretty smart that if you target one, they’re going to find a way to get themselves around that medicine you’re giving them. They’re quite ingenious and able to grow despite our single treatments. Each myelofibrosis patient is very different so identifying what the appropriate second agent would be is really on a case-to-case basis.
Part of the challenge that we have with medicines against blood cancers or cancers is finding ways to deliver medicines solely to the cells affected by the disease without harming normal cells.
Dr. Ruben Mesa
Ruth: What’s exciting to see is that we’re more forward-thinking in our endpoints of clinical trials so we’re looking more at survival and not just symptom relief.
All of the clinical trials that we’ve talked about so far are exploring new drug treatments. There are also trials that look at lifestyle issues like exercise or the benefits of yoga and meditation.
Dr. Fleischman is renowned for her work studying inflammation and diet. She and her collaborators recently published the results of a clinical trial that tested the feasibility of the Mediterranean diet for MPN patients.
For those of us living with an MPN, anything we can do ourselves to potentially control our symptoms is very welcome. In fact, it helps us feel in control of our own blood disorder. Even if it’s only a little bit, it’s important.
Dr. Mesa, let’s move on to an area of treatment that the MPN community is very excited about exploring, which is a novel therapy targeting calreticulin, the CALR mutation, one of the most common drivers of essential thrombocythemia and myelofibrosis. People are calling this a potential vaccine. What can you tell us about this that explains it best?
Dr. Mesa: About a third or more of patients with myelofibrosis have a mutation in the protein called calreticulin. This is a little different from JAK2 and MPL because calreticulin might be on the surface of the affected cells and we may be able to target the abnormal calreticulin in a specific way.
Part of the challenge that we have with medicines against blood cancers or cancers is finding ways to deliver medicines solely to the cells affected by the disease without harming normal cells. In the bone marrow, that’s particularly important.
There has been a great interest in seeing if we can target calreticulin in a specific way. A vaccine trial has been done in Europe and is in its infancy.
There’s a theme of multiple different approaches being developed, both cellular-based therapies, such as CAR T or vaccines, or a monoclonal antibody against calreticulin. That is creating great interest but has not yet started in human trials. In the laboratory, it appears to be a very promising medicine.
We’re very hopeful, but we also recognize that until we’ve started treating patients, we never know how good a fit it’s going to be, if there’s going to be an unexpected side effect, or if it will have the benefit that we hope that it has.
I certainly hope that this medication is successful. Even if it is not, I suspect there will be others behind it that hopefully will be. We learn from trials whether they end up being a home run or not. This theme of us trying to selectively target the cells involved is really key.
As we think about anemia, there are JAK inhibitors that can have an impact on the spleen and symptoms, but they may either worsen anemia or not improve anemia.
Dr. Ruben Mesa
Treatment for Patients Who Suffer From Anemia
Ruth: Dr. Mesa, as we know, anemia is very common in myelofibrosis and many people need regular transfusions so this isn’t something that’s likely to go unnoticed. There can be severe fatigue associated and MF patients will have regular blood tests so in theory, it should be seen and diagnosed early on. Tell us about some of the new hopeful treatment options for MF patients who suffer from anemia.
Dr. Mesa: Anemia is when an individual has fewer red blood cells than they’re supposed to. Red blood cells carry oxygen from our lungs to the rest of the body so that’s key for us in terms of our muscles to work well and for us to feel well. That’s why we can have issues in terms of anemia.
Myelofibrosis anemia has multiple aspects. There can be baseline anemia because the bone marrow is not producing enough red blood cells. There can be other contributors if they’ve had baseline iron deficiency that contributes to that. If the spleen is very enlarged, it can hold on to many red blood cells.
Historically, we’ve had a range of options. None of them have been overly dramatic in terms of their impact, but they can help. Injections such as erythropoietin-stimulating agents and androgens like testosterone help to produce red blood cells in both men and women.
There are medicines that are in development. Luspatercept, a medicine that can help with anemia, is currently in phase 3 clinical trials for patients with myelofibrosis. Momelotinib, which was most recently in September 2023, is a JAK inhibitor that can also help to improve anemia.

We look for that medicine to improve both spleen symptoms and anemia. As we think about anemia, there are JAK inhibitors that can have an impact on the spleen and symptoms, but they may either worsen anemia or not improve anemia. Both things can be present.
Momelotinib is an important advance. I hope that as we see other combination therapies, we may see broader improvement in spleen symptoms and anemia.
Should momelotinib or those agents that are more helpful with anemia, perhaps even pacritinib, be that JAK inhibitor to be used with other medications in combination? That has yet to be answered with some of these combinations.
Some real progress in anemia and improving anemia can clearly have a benefit, in terms of the patient’s quality of life and their ability to have enough energy to do their daily activities or things they enjoy, as well as being easier on the body.
One area that we’re intensely working on is trying to identify surrogates for survival or something that we can capture with data amenable to a clinical trial that will be a marker for disease impact.
Dr. Angela Fleischman
Symptom Control While Being on Treatment
Ruth: Dr. Fleischman, what I find very exciting in today’s clinical trials is that we’re moving beyond symptom management and moving more toward progression-free survival, living longer with a high quality of life. When evaluating clinical trials, it’s important to understand the goal of the study. Where do you think we are with this?
Dr. Fleischman: That’s a very important point. For a clinical trial, you need to have an endpoint, a defined thing that you’re going to test. Ideally, something that you anticipate that your drug of interest is going to achieve better than the comparator.

Classically, in myelofibrosis, we’ve focused on spleen volume reduction and symptom burden because those are two things that we can quantify and expect to see a change in a short period of time. Although spleen size reduction and symptom burden are extremely important, they just don’t capture the whole picture of myelofibrosis.
The endpoint of survival obviously is extremely important to myelofibrosis patients. Survival is extremely important to everybody. But because, in general, myelofibrosis patients live a long time, it’s not a feasible outcome for clinical trials.
One area that we’re intensely working on is trying to identify surrogates for survival or something that we can capture with data amenable to a clinical trial that will be a marker for disease impact.
There are some clinical trials such as the imetelstat clinical trial in which survival is the actual endpoint, which is a unique and forward-thinking endpoint. One of the key areas of need in myelofibrosis is identifying markers that can be used in clinical trials that indicate a significant impact on the disease that goes beyond reducing spleen size and symptoms.
Ruth: We’re definitely getting into a very exciting time that will make such a difference therapeutically once we have those answers.
A transplant itself is an extremely risky procedure that can lead to significant side effects or even death.
Dr. Angela Fleischman
The Best Time to Get a Stem Cell Transplant
Ruth: When is the best time to get a stem cell transplant? I know there isn’t a black-and-white answer, but Dr. Fleischman, how do you answer this common question?
Dr. Fleischman: That is another million-dollar question in myelofibrosis and I think the most difficult decision for a myelofibrosis patient as well as for the physician.
As we know, at this point in time, a bone marrow transplant is the only curative option for patients with myelofibrosis. You may say, “If it’s curative, why doesn’t everybody just get a transplant?”
Transplants themselves can be quite risky. The media may miscommunicate transplants. You always see on the news that somebody’s looking for a donor so it may seem that the only problem with transplants is you can’t find a donor. That’s not necessarily the case.
A transplant itself is an extremely risky procedure that can lead to significant side effects or even death. We don’t want to give somebody a transplant if the transplant is going to make them sicker or potentially lead to their death earlier than would be the case if they just treated their myelofibrosis through other means.

The ideal time to transplant is when you get clues from this myelofibrosis patient that with standard treatments, they are very likely going to have an extremely bad outcome or will have an outcome that will lead to their death very quickly. That’s the time going forward with a transplant is worth the significant risk.
The safer the transplant becomes, the greater the consideration of when it is used.
Dr. Ruben Mesa
Dr. Mesa: There is a balance between a patient undergoing a stem cell transplant and a medical treatment. They have very different dynamics. A stem cell transplant can potentially be curative but can come with significant risks, including upfront risk.
The benefits of medication depend on many things, like how much benefit, how long is that benefit, and the impact on the patient. I would certainly view it as a success if we are able to have medicines that are sufficiently impactful that it leads us to either be able to skip a bone marrow transplant or delay it further.

We have precedent in other areas and the most successful example is in chronic myeloid leukemia. At the beginning of my career, most young patients had a stem cell transplant. Now, with the impact of medications, it is rare for patients to have a stem cell transplant because of the effectiveness of medications.
Currently, we are not there yet. A patient who clearly needs a transplant still will go for a transplant. Over time, there may be more combinations of both. Can the medicines help to decrease the burden of the disease and make the likelihood of success with the stem cell transplant greater? That is also a win.
In parallel, there’s tremendous research from our colleagues who do stem cell transplants to try to make that process safer. The safer the transplant becomes, the greater the consideration of when it is used.
It continues to evolve. We reevaluate every option we have. Does that change the dynamic in terms of transplantation as well as how the two things work together? With our current medicines, we are not changing our decisions for transplant, but increasingly, almost all patients that go to transplant have had at least one or more of the JAK inhibitors ahead of time.
The data would suggest that the outcomes with transplant are better in part because of JAK inhibitors but also because of improvements in the processes, antibiotics, other medications, and expertise of our colleagues who run transplant centers.
Asking About Clinical Trials
Ruth: When should a patient ask about clinical trials? How and when do you introduce the idea of considering a clinical trial to your patients?
Dr. Mesa: Clinical trials may exist in any aspect of the disease, from diagnosis to initial treatment to treatment downstream. It’s always a fair question for patients to ask. Is there a clinical trial that may be appropriate for me? It is not limited to one segment of the disease.
Clinical trials are how we make progress. They are highly regulated and always have the needs of the patient front and center. At a minimum, a patient is always getting the option that is at least as good as the standard of care.
It’s a fair question to ask at any point along the way. Truly, all the options we currently have have only been possible because of patients’ willingness to participate in clinical trials.

As physicians, we are happy to have another set of eyes look at the patient and hear another person’s thoughts. That is in no way hurting your physician’s feelings by seeking out a second opinion.
Dr. Angela Fleischman
Dr. Fleischman: This may be a different answer than one may get in a community practice. When I approach a new patient and we talk about treatments, I’ll always talk about standard-of-care treatments first and give people their options, and the pros and cons of each of the standard treatments.
Afterward, we’ll discuss potential clinical trials that the patient might be eligible for. I talk about clinical trials at my own university or elsewhere that I think would be appropriate for the patient. If there was something specific that I thought the patient would be ideal for, we’d talk about that trial first.

Because I want to give the patient all of the information, I would talk about other clinical trials that the person may be eligible for. I would give them reasons why they might be a good idea but also why I don’t think that clinical trial would be ideal for them and give them specific reasons why.
I highly recommend second opinions or even third opinions. Sometimes patients feel bad going for a second opinion. Their primary oncologist may think that they are not happy with their approach. That is not the case.
As physicians, we are happy to have another set of eyes look at the patient and hear another person’s thoughts. That is in no way hurting your physician’s feelings by seeking out a second opinion.
I highly recommend a second opinion or a third opinion, even if it’s just, “I want to learn more about my disease. I’m happy with my treatment now, but I want to get somebody else’s point of view, learn about my future, and what might the options be if this happens or that happens.”
All drugs must move through the steps or phases to ultimately be approved by the FDA.
Melissa Melendez
Trial Knowledge #1: Phases of Clinical Trials
Ruth: Melissa, clinical trials try to see if we can improve the standard of care and give more and better options to more people.
There are different phases of a clinical trial. What are they and what do they mean?
Melissa: I like to think of clinical trials as steps to drug approval. All drugs must move through the steps or phases to ultimately be approved by the FDA. If drugs are studied in new combinations or in diseases outside of their approved indication, they still need to go through all the phases.

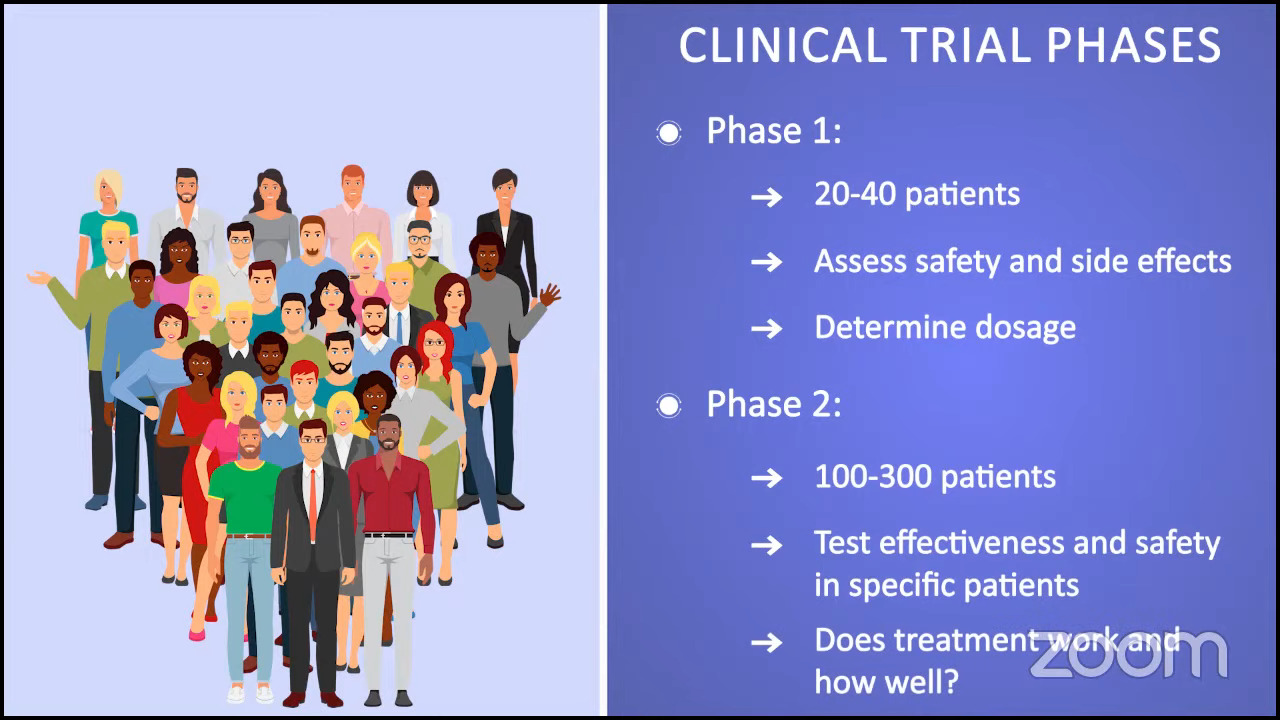
For phase 1 trials, the treatment is tested in a very small number of patients. It could range from 20 to 40 patients. We’re testing dosage and looking at the safety and side effects.
Phase 2 trials typically build on phase 1 results. They try to determine the effectiveness and safety of the drug in a specific population. We’re trying to answer the questions of whether a treatment works and how well it works.
For example, there may be a phase 1 trial that tests a drug in blood cancer patients or MPN patients. After the phase 1 results, they determined that myelofibrosis patients showed the best response to the drug. The phase 2 trial now might only include myelofibrosis patients and they’ll be testing it in a larger subset, like 100 to 300 patients.
Researchers continue to monitor patient safety throughout the phase 2 trial and phase 2 studies with positive results will then move into the phase 3 trial.
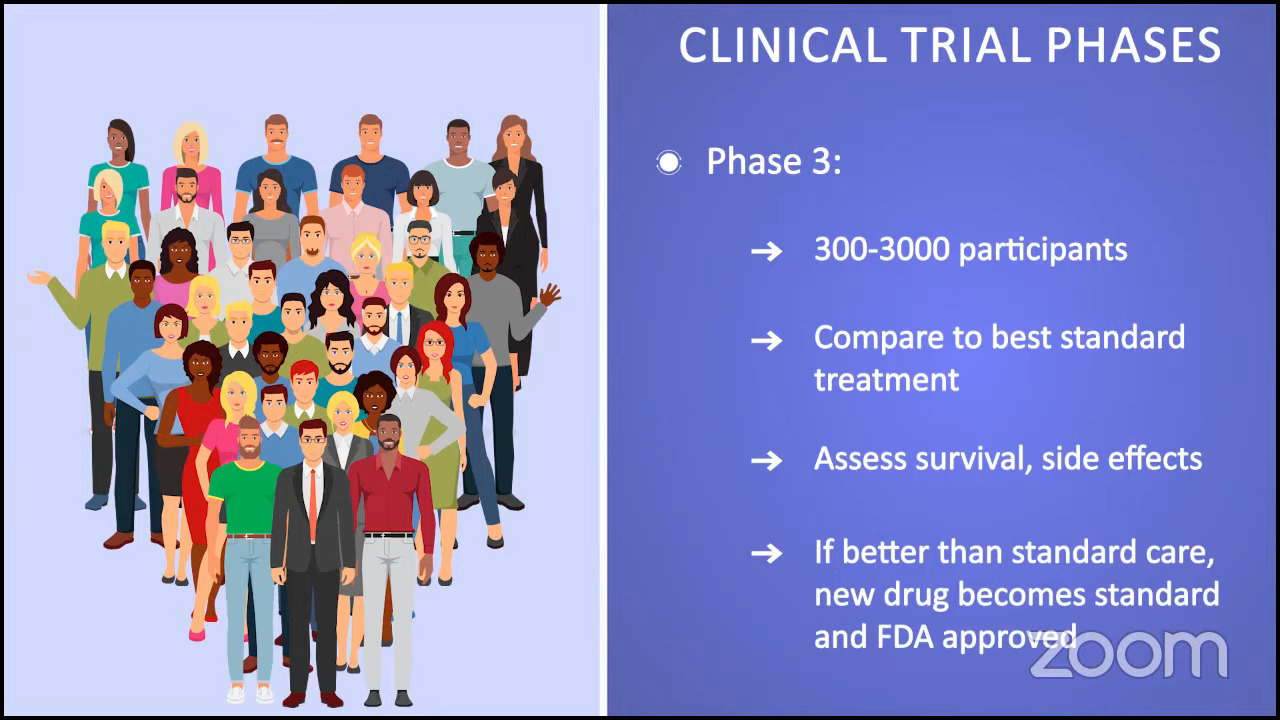
Phase 3 trials are often referred to as randomized trials, comparing a new treatment against the best standard treatment. These trials can be done with anywhere from 300 to 3,000 participants. Researchers are trying to see if a new treatment has better survival outcomes and fewer side effects.
When a trial is completed and shows that the drug is in fact better than the standard of care or the best standard treatment, that’s when the newly investigated drug becomes a standard of care and will become FDA-approved.
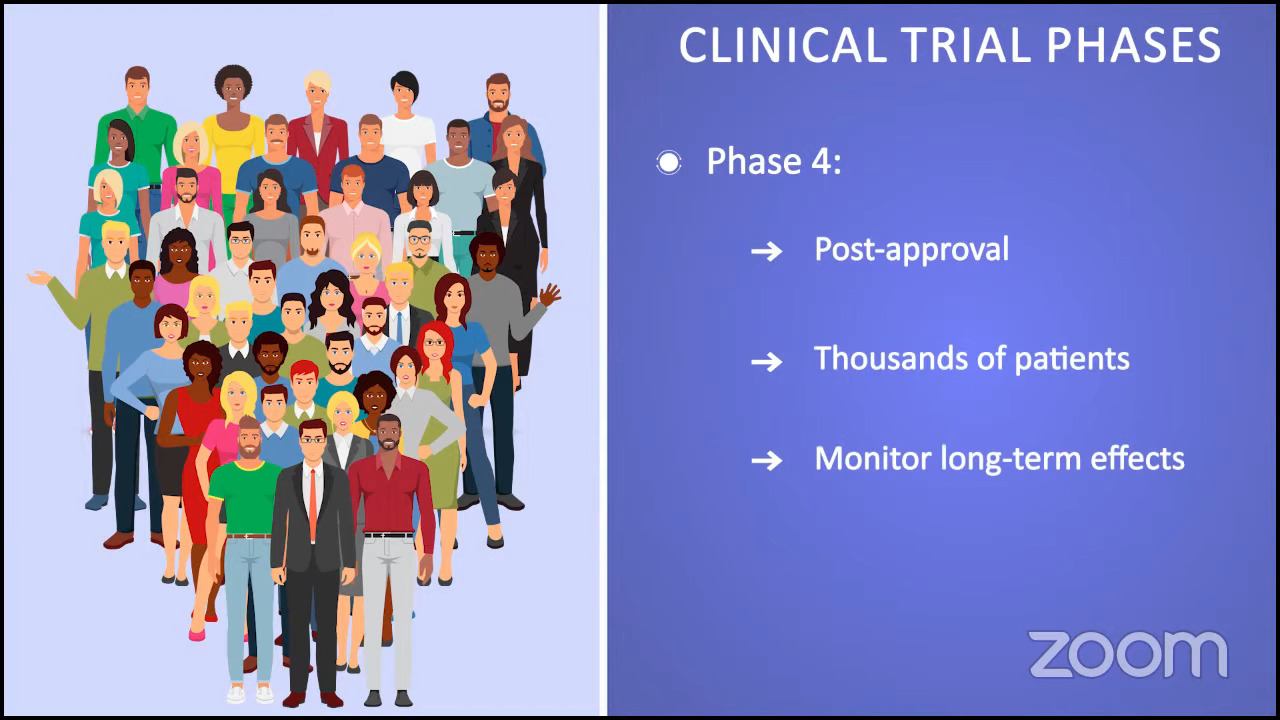
By the time a clinical trial enters phase 4, the FDA has already approved the treatment. These trials are done in thousands of patients and they usually go on for many years.
Placebos are not typically used with patients in cancer clinical trials. But if a placebo is used, the person will also receive the standard treatment for their specific cancer.
Melissa Melendez
Trial Knowledge #2: Use of Placebos in Clinical Trials
Ruth: This one needs myth-busting. A lot of patients and care partners say, “I wouldn’t want to risk getting a placebo.” Are placebos used in myelofibrosis clinical trials?
Melissa: Ruth, I’m really glad you asked that because federal regulations require patients to know if a placebo, which is an inactive substance that looks the same as the one used as treatment, will be incorporated into a trial. An example of a placebo could be a sugar pill.

Placebos are not typically used with patients in cancer clinical trials. But if a placebo is used, the person will also receive the standard treatment for their specific cancer. In our case for myelofibrosis patients, the placebo will be in combination with active drugs so patients are receiving at least the standard of care. No one with active cancer would be treated with only a placebo because that is unethical.
When researchers do use a placebo, they must tell the patient that they have a chance of getting a placebo and that they will receive an experimental treatment at some point in the clinical trial, if not right away. For example, you may be assigned to the placebo group, but if your cancer gets worse, researchers will switch you to a study drug or new treatment.
The more you ask and open up the lines of communication with your team about potential treatment options, the more you learn.
Melissa Melendez
Trial Knowledge #3: Finding Clinical Trials
Ruth: For people who want to explore clinical trials, what can they do to find one for themselves? The ClinicalTrials.gov site isn’t the most user-friendly and that’s why the LLS Clinical Trial Support Center is in existence and is so important. Tell us about that.
Melissa: I agree that ClinicalTrials.gov isn’t the most user-friendly and can be difficult to use. ClinicalTrials.gov is a searchable registry and database of clinical trials conducted in the United States and around the world. It can be overwhelming and this is where the patient’s healthcare team and the CTSC can come into play.
Patients can talk to their doctors and learn more about clinical trials done at their home institution or outside of it. The more you ask and open up the lines of communication with your team about potential treatment options, the more you learn. This can help you better understand what options are available to you and feel more comfortable moving forward with a treatment plan.
The LLS created the Clinical Trial Support Center to arm patients with information to take back to their healthcare team. The CTSC provides a free service to patients. It usually takes under five minutes to fill out a form online. Don’t get overwhelmed. If you’re worried that you haven’t filled out enough information, please submit it anyway.
We reach out to patients within 1 to 2 business days; typically, on the same business day that we receive the referral. When you connect with one of the nurses, you can expect a teammate who will join you on your cancer journey.
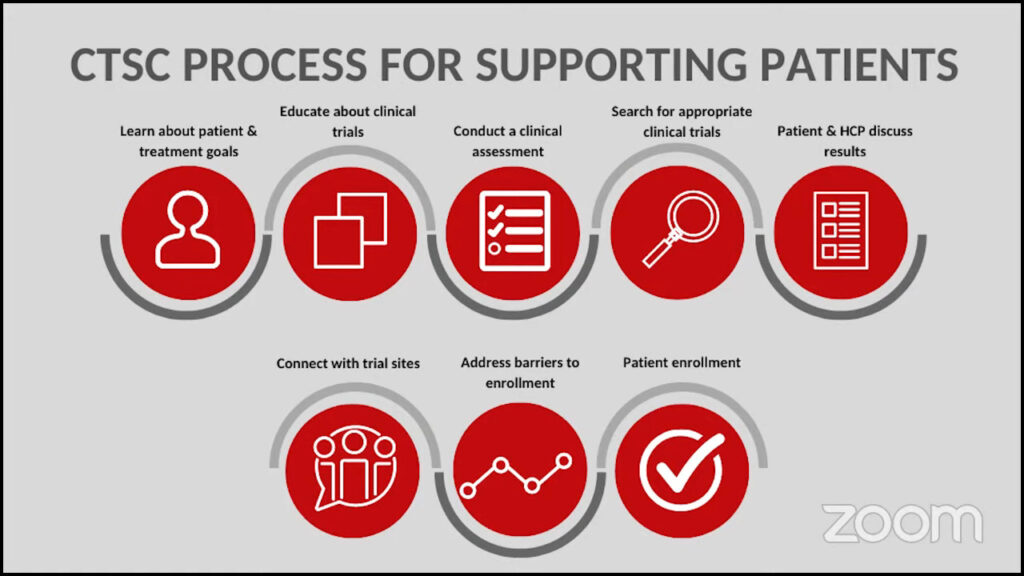
We take your individual needs into account. We learn about your treatment goals. We educate the patient about trials and conduct a clinical trial assessment. This includes addressing barriers, such as travel, and discussing financial assistance through LLS or even outside organizations.
We search for trials and go through each one individually, which is a little bit different than other online services. We take an individualized approach and only send trials to patients that they’re likely eligible for. We send the results to the patient and encourage them to take them back to their doctors to discuss the results.
We provide patients with a non-biased, patient-friendly list of appropriate clinical trials. Ultimately, we work in collaboration with the patient’s healthcare team to decide if a clinical trial is right for them. We offer to reach out to the trial sites if there’s anything on the trial list that they would like to learn more about.

We have many patients that we’ve worked with for several years and developed a personal connection with them. We really strive to provide continuity of care throughout their journey. I always tell my patients that I’m just a phone call away and they can always reach out to me at any point with questions or if their disease or treatment changes.
Over time, trials may change. Additional trials may open up or some may have closed so I want to make sure that I can provide them with an updated search any time.
Our services are available to myelofibrosis patients as well as other blood cancers ranging from pediatric patients to adult patients. We even encourage healthcare providers to reach out to us. We do clinical trial searches for physicians in large centers and smaller community centers where they don’t have as many resources or staff.
The clinical trial landscape is pretty complicated. CTSC nurse navigators are here to break down the barriers to clinical trials that patients face to help them make informed decisions. We’re here to help and welcome any patient referrals.
We learn about each patient and take a personal approach to find out their treatment goals, their physician’s goals, and what they’re looking for in a trial.
Melissa Melendez
Trial Knowledge #4: Staying in a Clinical Trial
Ruth: What are the top three things that you do at LLS to help people stay in a trial? Talk about the challenges and the solutions.
Melissa: We learn about each patient and take a personal approach to find out their treatment goals, their physician’s goals, and what they’re looking for in a trial.
Barriers are the first challenge. Nurse navigators are proactive during those conversations on breaking down barriers that patients might face. Are these barriers financial? Will they have to travel if we find one that they’re interested in? Our solution to these issues is to find out if a trial offers financial assistance in addition to what we may offer at LLS.
We email trial coordinators and principal investigators and inquire about the treatment schedule. How many visits may the patient expect? Can they get labs at their home institution?

This is also dependent on what phase the trial is in. This may not be possible for phase 1 trials, but more possible for phase 2 or phase 3 trials. Can any of the long-term follow-up visits be virtual?
Communication is also another challenge. Our solution in the CTSC is to help break down the intimidating medical language or trial jargon. We try to use the simplest explanations and if patients want more in-depth or complex information, we can definitely adjust and provide that as well.
We always check on a patient’s understanding. When we’ve explained something or provided information, we may ask the patient to repeat what they understood and listen as they walk through that process with us. The more they understand, the more comfortable they feel when they’re making the decision.
Trial awareness is a challenge and we can help raise awareness through programs like this. We talk about trial myths and help patients understand the clinical trial landscape better. We help patients be more well-informed about trials that may be available to them and about personalized trial services like ours and the Clinical Trial Support Center at LLS.

At the LLS, our main hub is our information resource center through our information resource specialists, nurses, and social workers. You can call the 1-800 number Monday through Friday, 9 a.m. to 9 p.m. Eastern Standard Time.
We also have a chat option where you can do a live chat online, and that’s Monday through Friday, 10 a.m. to 7 p.m. Eastern Standard Time.
If for some reason you reach out to us outside of our business hours, you can always leave a message 24/7 and we’ll give you a call back.
We’re in a very exciting time for myelofibrosis… We can personalize FDA-approved JAK inhibitors for each myelofibrosis patient.
Dr. Angela Fleischman
Final Takeaways
Ruth: If you could leave people with just one message, what would that be?
Dr. Mesa: I would leave them with a message of hope. We have made a tremendous amount of impact and a much greater understanding of the biology of the disease, the genetic mutations, and why people progress. We are building on a base of very good medicines that have made an impact with the JAK inhibitors but to a new era of multiple different approaches.
I would heavily encourage patients to consider clinical trials where appropriate. Clinical trials will build on the base of these medications to try to drive progress further and further. We need your help and together we will continue to improve the therapy of diseases like myelofibrosis.
Dr. Fleischman: We’re in a very exciting time for myelofibrosis. In the past few years, we’ve moved from a single FDA-approved JAK inhibitor to four JAK inhibitors. We can personalize FDA-approved JAK inhibitors for each myelofibrosis patient.
There are a lot of opportunities for combination treatments that are moving forward in clinical trials as well as going beyond JAK inhibitors, trying to identify other key pathways to target myelofibrosis.
Melissa: In the CTSC, we educate, support, and empower myelofibrosis patients as well as other blood cancer patients to be active participants and have control over their treatment decisions with their healthcare team.
The Leukemia & Lymphoma Society wants to make a difference in patients’ lives through research, advocacy, education, support, and financial assistance. We want to see a future without blood cancers. We have a lot of free information for patients, caregivers, and healthcare providers to check out.
We’re a phone call away and we are here to help make clinical trials less overwhelming for patients and to help healthcare providers.
Ruth: We have covered an awful lot of information and invaluable insight from our experts. I know I speak for people living with ET, PV, and MF, their loved ones, and care partners when I offer our very sincere gratitude to you all for everything you do for our community.
Stephanie: Thank you so much, Ruth. I always appreciate the way you bridge the conversation for us.

Thanks so much to Drs. Mesa and Fleischman for the work that you do in the clinic and in research to help patients and families who are trying to navigate a myelofibrosis diagnosis and treatment, and all the decisions that come in between.
Thank you to our LLS clinical trial nurse Melissa Melendez for being there day to day and helping people who are overwhelmed by the topic of clinical trials.
We really hope that you walk away with a greater understanding of clinical trials in myelofibrosis. We hope to see you at a future program.

Special thanks again to GSK for its support of our independent patient education content. The Patient Story retains full editorial control.





