Living Well Together with Follicular Lymphoma: Real Talk from Patients & Partners
How do you navigate living with follicular lymphoma (FL) as a couple from diagnosis, to treatment decisions, and life beyond?
In this heartfelt discussion, three couples share their personal stories and unique insights on living with FL. From the shock of diagnosis to finding emotional balance, hear real talk on what helped them move forward together.
Real Talk from Real People: Three couples share how FL changed their lives and how they’ve supported one another through it.
Patient-Care Partner Dynamics: Explore the emotional impact of FL from both perspectives.
Honest Reflections: Hear about moments of fear, uncertainty, resilience, and love.
Lessons Learned: Gain practical insights about advocating for yourself, communicating with doctors, and managing the emotional weight of the journey.
Inspiration and Encouragement: You are not alone in this. See what healing, partnership, and hope can look like.
We would like to thank The Leukemia & Lymphoma Society and the Living with Follicular Lymphoma Facebook Group for their partnerships.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make treatment decisions.

Thank you to Genmab for its support of our independent patient education program. The Patient Story retains full editorial control over all content.
Edited by: Katrina Villareal
- Introduction
- What Were Your First Symptoms of Follicular Lymphoma?
- Getting a Doctor to Address Your Concerns
- Helping a Patient Cope as a Care Partner
- Processing Your Emotions as a Care Partner
- Major Challenges Faced by Care Partners
- Deciding on the Right Follicular Lymphoma Treatment
- How Care Partners Can Help with Treatment Decision-Making?
- Biggest Emotional Challenges for Couples
- Biggest Lessons Learned as a Patient and Caregiver
- Conclusion
Introduction
Stephanie Chuang: The focus for today is living well with follicular lymphoma, navigating the healthcare system, staying informed and connected in the system, and so much more. We’ve got an incredible panel.
I’m the founder of The Patient Story and I had my own blood cancer diagnosis. When I was getting my 600-plus hours of chemotherapy — so much fun — I felt very isolated and confused. I wanted to hear from real people, experts who are not already being highlighted, and to me, that would be other patients and care partners. That’s when the idea came up to help others navigate life at and after diagnosis, which is what The Patient Story aims to do. We try to build community through in-depth patient stories, educational programs, and discussions to amplify your voices, the voices of patients, caregivers, and partners.
Before we get into everything, we want to thank our great partners, The Leukemia & Lymphoma Society and the Living with Follicular Lymphoma support group on Facebook. The LLS provides free resources, including one-on-one support through their Information Specialists, who are available to answer your questions. The LLS also has a community section, a forum to chat with others living with follicular lymphoma.
Living with Follicular Lymphoma also provides another community where you can chat in real time with thousands of people living with FL from around the world. It is the largest FL-dedicated Facebook group online.

We would like to thank Genmab for its support as our sponsor, which allows us to continue hosting these discussions and programs free for our community. I want to stress that The Patient Story retains full editorial control and while we hope that this is helpful, remember that this is not a substitute for medical advice. Please consult with your team as you’re making your decisions.
Joining me are follicular lymphoma patients and their care partners. We have Nicky and Craig from Perth, Western Australia, Hayley and Carl from St. Louis, Missouri, and Milissa and Andrew from Clear Spring, Maryland. I’m joining from just outside San Francisco, so we’re covering a lot of space.

Nicky & Craig
Nicky: I was diagnosed with follicular lymphoma in February 2014 at 32 years old. My kids were very young then, one and four. I also recently graduated from university and I’m now a clinical nutritionist. My passion is supporting cancer patients through their nutrition and lifestyle.
Craig: I’m Nicky’s husband and we’ve been together for a long time.
Nicky: 28 years, I think.
Craig: I’m Nicky’s caregiver, but it’s probably a lot more than that.
Nicky: We’re each other’s caregiver.
Craig: I’m a dad, I work, and I try to keep everything rolling.
Milissa & Andrew
Milissa: I was diagnosed with follicular lymphoma at the end of 2023. Andrew and I had been living together for a little over a year at that time. I’m also a nurse, so that put a different spin on everything that went on.
Andrew: Milissa and I have been together since August 2021 and have been living together since September 2022. She was diagnosed with lymphoma in November or December 2023 and started getting treatment in January 2024. It’s been a challenge since she seeks treatment in New York City, which is about a five-hour commute from where we are. But she gets good care where she goes, and right now — knock on wood — she’s doing well.


Hayley & Carl
Hayley: I got diagnosed with stage 3B follicular lymphoma in October 2024, which had already transformed to diffuse B-cell when we found it. We started treatment by November. I’m also a nurse and work in the emergency room, so that’s where I got my diagnosis, at work.
Carl: We’ve been together nearly two years. We’ve been together about a year and a half when she was diagnosed. Luckily, we had already lived together when that happened. I have two kids, so my role was to support her and try to stay healthy and present. I’m lucky that I work remotely, so I’ve been able to attend almost every appointment, be there for her as best as I can, and try to hold everything together. She’s done great and we’re happy to see the other side of this.
What Were Your First Symptoms of Follicular Lymphoma?
Stephanie: Let’s kick off with when you found out about all this. We know that for follicular lymphoma, many people don’t feel any symptoms, but some feel subtle symptoms, so there’s a bit of a range there. A lot of people get curious about this part of the story. How did this lead to a diagnosis? Nicky, you were diagnosed at 32. I was 31. We both mentioned fatigue, which, by the way, isn’t just tired. It’s beyond that. But you also just had your son, so there’s a feeling that the fatigue comes with that, doesn’t it? What moved you to become concerned?
I felt my neck and realized that it was like a corncob.
Nicky
Nicky: I didn’t notice any signs or symptoms. I was working, I just had a baby, and I had a toddler as well, so I thought that being tired and fatigued was normal. I used to be a mortgage broker. One of my colleagues was a bit of a hypochondriac who always thought she had any kind of illness that came. She mentioned that she always checks her neck for lumps because in her head, if you have a lump on your neck, it means that you have cancer.
I laughed it off, but I felt my neck and realized that it was like a corncob. It was very lumpy. I didn’t worry too much, but I went to the general practitioner (GP) and he said, “Oh, okay, we’ll do a blood test and see.” I never heard back. I went back about a month later and told him I still had lumps on my neck, and that’s when he said to do some more testing. It took a good two to three months for me to get my actual diagnosis. There was a lot of back and forth going to different doctors and getting tests done, so that was a little traumatic at the time.
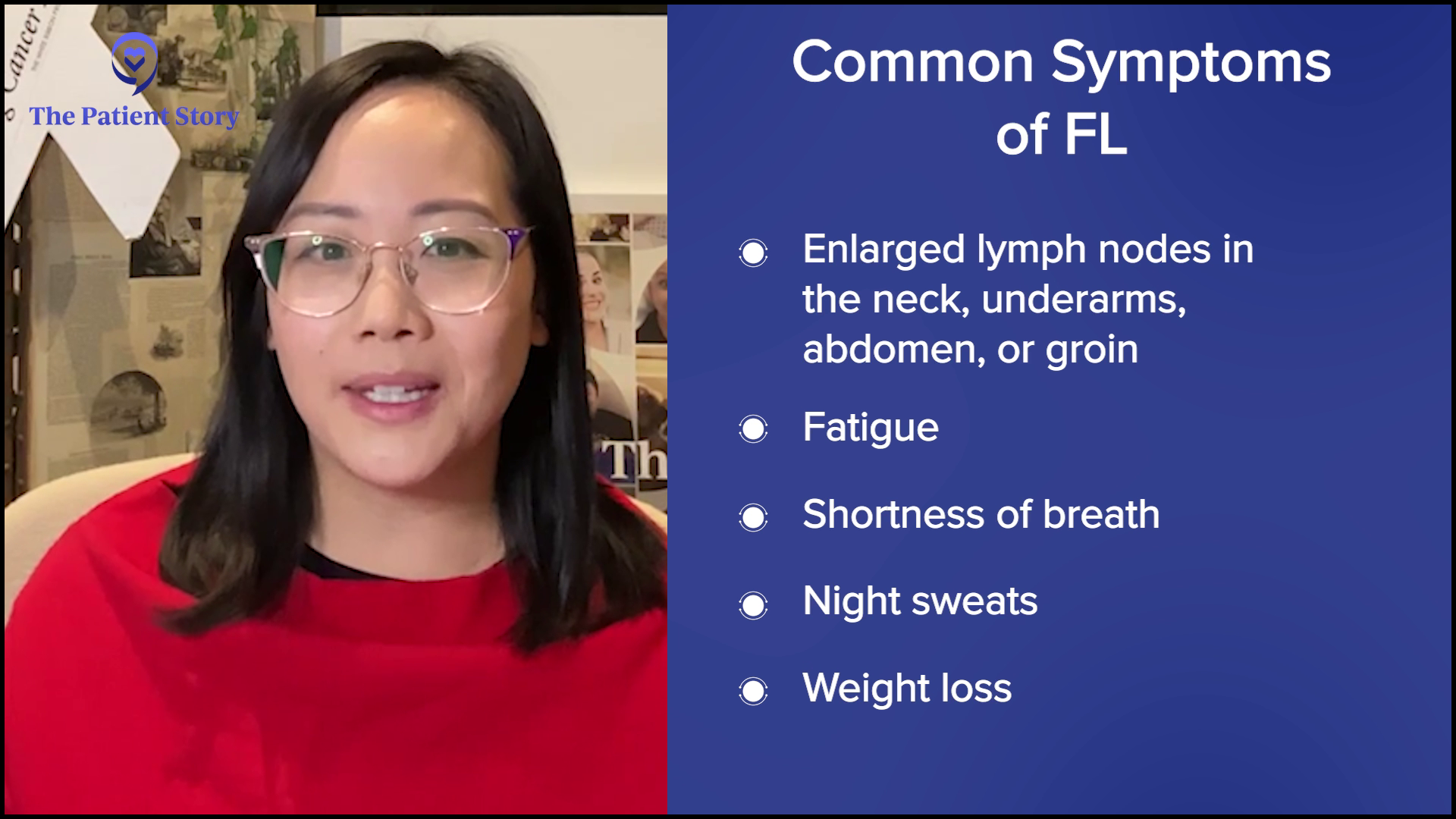
Stephanie: Hayley, you shared that you felt pressure in your neck, but it didn’t feel like a typical sore throat and you were otherwise feeling healthy. What pushed you to listen more to your body and to seek medical help?
When I would sit slouched back on the couch and breathe out a few times, I felt a wheeze.
Hayley
Hayley: It originally started with a deep-set lump over my clavicle. It didn’t feel like a lymph node; it almost felt like a pulled muscle. It wasn’t bothering me all day and it wasn’t even every day. It was only in certain positions. When I would raise my arms above my head, I would feel the pressure. Now, looking back, I know exactly what that was. Every time I lifted my arms, it would push the mass. It deviated my trachea.
Besides those nodules, my only other symptom was a little bit of wheezing, which I didn’t think anything of at the time. It wasn’t alarming enough or often enough to spark concern. When I would sit slouched back on the couch and breathe out a few times, I felt a wheeze. I thought I only needed to change my position. I found out that I had a big mass right in the middle of my chest and that’s never happened since then, so now I know that’s what that was.

Getting a Doctor to Address Your Concerns
Stephanie: When you were seen by a doctor and voiced your concern, did you get any feedback like it’s nothing? Especially as young women, sometimes we hear stories about that.
Hayley: That morning, I called an ENT because at that point, I knew that it wasn’t something normal, and it came on overnight. I said, “I would like to get a scan.” The ENT said, “I’ll see you in six months,” and I said, “It came overnight, so in six months, I don’t think I’ll be here.”
When I went to my primary care doctor, she was pushing on my thyroid and having me swallow. She wasn’t quick to order a scan. I had to push for that. She was thinking along the lines of a thyroid issue and ordered an X-ray. Originally, the only thing that we had was the X-ray. They called me that night with the results showing widening of my mediastinum. I was shocked because I was under the impression that I might have some swollen nodules. I didn’t understand why she ordered an X-ray of my chest.

Stephanie: I’m so glad you pushed for what you needed. As a nurse, I can only imagine all that you know when it comes to this. Milissa, did that also show up for you in terms of self-advocacy, in trying to figure out the diagnosis? Did you have any red flags for symptoms that you experienced?
Milissa: I commonly had lymph nodes in my neck. I went to primary care doctor who also thought it was my thyroid. They did some ultrasounds, but they didn’t see anything. They did a CT scan and it came back with “possible lymphoproliferative disease.” I went to see an ENT doctor at a pretty big center and he said it was just inflammation. He didn’t know what it was, told me to get another ultrasound of my thyroid, and then said, “I’ll see you in six months.” I said, “Can we biopsy it?” And he said, “No, I don’t think it’s warranted.” I went on for another two years until I got another CT scan.
Stephanie: We’ve heard that sometimes, it does take time, especially when there’s not a more pressing symptom to flag something.
It’s a little hard to grasp the severity of it. You don’t have a lot of answers in the beginning.
Andrew
Helping a Patient Cope as a Care Partner
Stephanie: There are two very different perspectives when we enter a diagnosis: the person who’s diagnosed and the main care partner. For each care partner, what do you think your partner was thinking and feeling in terms of how to handle the diagnosis?
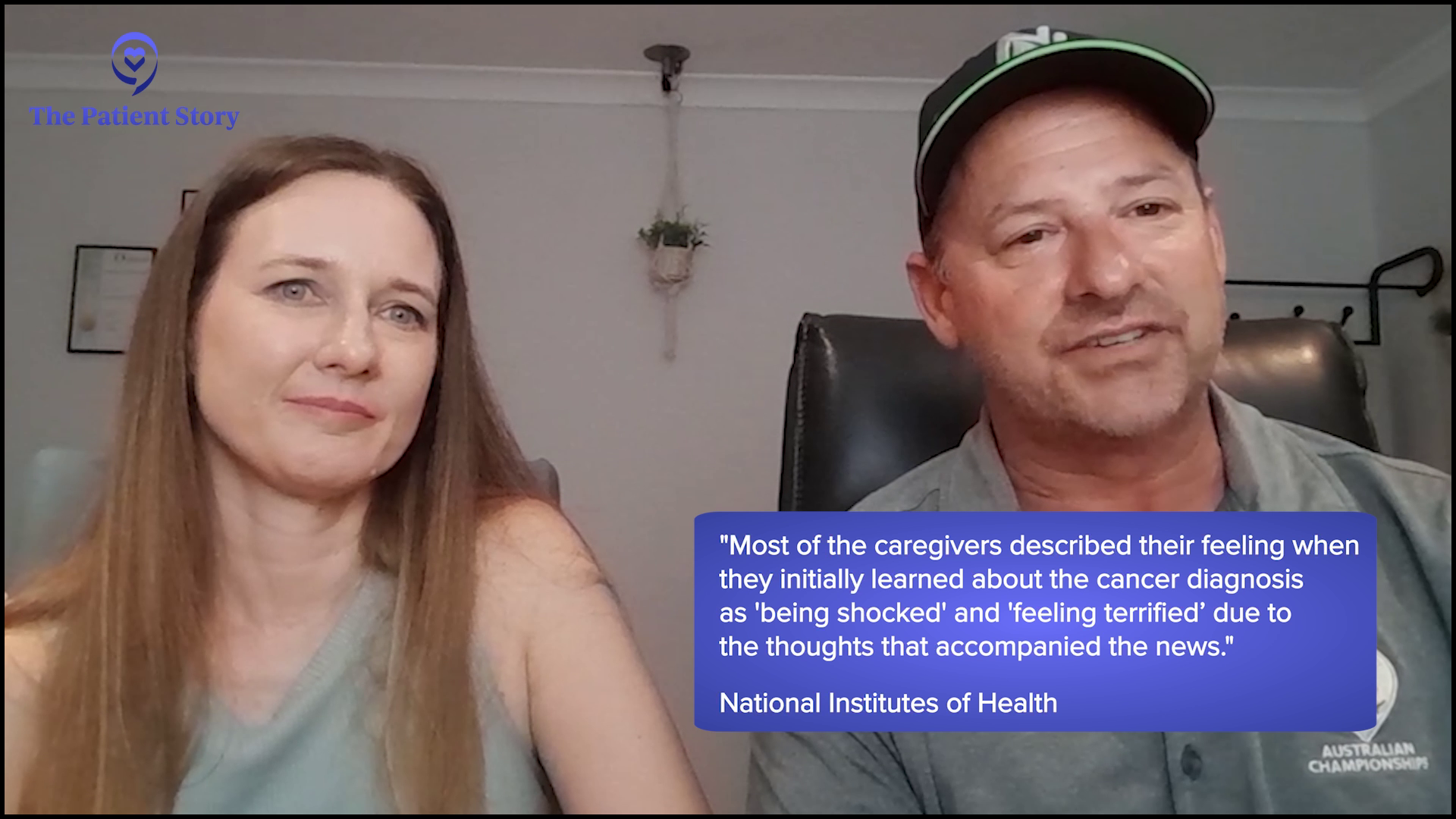
Andrew: It was a big shock for both of us. She’s always been very healthy, vibrant, and energetic, so you’re thrown back by it. It’s a little hard to grasp the severity of it. You don’t have a lot of answers in the beginning. Milissa knew that she needed to get more answers and she wanted to go to a facility where she would probably have the best chance of getting the best treatment. Being a nurse, she wants to research and look up symptoms, which is good because she’s educated in that respect and I’m not.
Stephanie: Milissa, what I heard was part of it was fear but being in the field of healthcare, you know a lot, which is good, but it brought some anxiety. When you heard him describe your reaction, was that spot on or is there anything else you’d like to add about what you were feeling?
Milissa: Yeah, that was spot on. I went down every bad road that there was.
I was feeling anxious about it and didn’t know what to do… If you’re diagnosed with cancer, your outlook on your future changes dramatically overnight.
Andrew
Stephanie: If you could list a couple of words for Andrew and how you think he was at diagnosis and moving forward into this new relationship, what would that be? How would you describe that?
Milissa: I think he was lost and terrified. He took one day at a time, whereas I took five years in advance, so it was probably better for him.
Stephanie: Andrew, did she nail that, or were there other things that you were feeling?
Andrew: No, I think she nailed it. I’m sure I was feeling anxious about it and didn’t know what to do. It’s a different role. If you’re diagnosed with cancer, your outlook on your future changes dramatically overnight. That’s hard to deal with and grasp right away for a person who loves you. It takes a little time to absorb those things.
Stephanie: Craig, could you talk about Nicky and her reaction and how she was during that time as you guys were trying to figure it out? What descriptive words would you use there?
Craig: Uncertainty, fear, being lost, and not knowing what tomorrow’s going to be. The information 10 years ago was a lot different compared to what it is now. It was very difficult. I don’t remember many days of my life other than my wedding day, but I do remember February 13th and the conversation. It was certainly a tough day.

Stephanie: Nicky, was there anything that Craig didn’t know at the time or did he pretty much capture all of that for you?
Nicky: It was pretty bang on. We live a little out of Perth and the doctor was a fair way away, so they called to give me the news. He was next to me and I said, “Is it cancer?” I didn’t know what lymphoma was. There wasn’t even that much information, but I remember the doctor said to me, “Google indolent.” Craig went off straight away and Googled indolent, then he came running back and said, “Don’t go on Google and don’t Google anything.” I was banned from Googling, but I don’t know what he read. It was a very shocking moment. We were very stunned because I didn’t expect to hear that. It was like a punch in the face.
Stephanie: When we talked, you mentioned the emotional response and that Craig felt something first, then when he was able to collect everything and move forward, then you got the chance to let go. Can you describe that?
Nicky: He came back and must have read something that scared him, so he broke down first. I said, “It’s going to be okay, love.” We called his mum to come over because she has breast cancer and we’re very close. When she arrived, that’s when it hit me and I broke down. We took turns supporting each other and as we started to get into it, the information came to light, but there was an initial shock.
After I had my bone marrow aspiration, we found out that it was stage 4. In our minds, hearing the words stage 4 for any cancer was the worst it could be, but for FL, it’s a bit different, which we didn’t know at the time. There’s a lot more information out there about FL than there was over a decade ago.
Craig: We went through the process before the diagnosis and went to numerous doctors’ appointments and procedures. I kept saying to Nicky, “You have nothing to worry about. It’s going to be fine. This is the process they go through and what they do to rule stuff out.” When we got the diagnosis, I felt awful that I was saying these things to her. I don’t know how to put it into words, but it was tough for me that I’d gone through that process with her, telling her everything’s going to be sweet, and then obviously it’s not. It’s like a bit of a bomb gets dropped.
Stephanie: Nicky, how is it hearing him describe that piece of it? Have you talked about that before?
Nicky: No, we haven’t spoken in depth about how he felt at the time of diagnosis, so it’s good to talk about it and get it out in the open, but I don’t think you should have any guilt about it. It’s nothing we could control. When you get a diagnosis like this, your whole mindset starts to change. Things that mattered before don’t matter anymore. Now I’m realizing what a blessing that is because it opened my eyes to take notice of things around me, make the most of every moment with our kids, and watch them grow. We don’t miss a moment anymore and I’m grateful for that.
Stephanie: Wonderful. Thank you both for sharing all of that. I know it’s a lot to remember back to 11 years ago, so thank you.
When you get a diagnosis like this, your whole mindset starts to change. Things that mattered before don’t matter anymore
Nicky
Stephanie: Hayley and Carl, I’m so interested to know if any of that resonated with either of you. Carl, what do you remember Hayley feeling and thinking at the time of diagnosis?
Carl: I was in the ER room when the doctor came in and gave her the diagnosis. I would say shock was the number one emotion, probably for both of us, because she was so young and healthy. We were active people, went to the gym quite a bit, and ate pretty well. Then it quickly turned to fear. Fear of the unknown and all the uncertainty, like Craig was talking about. She was quick to harness all that, bring it back, and hit everything head-on.
Carl and I had only been together for a year and a half, so I had a pit in my stomach. What was I signing him and his kids up for?
Hayley
Stephanie: Hayley, was there anything else that was going on for you? It wasn’t that long ago. Was Carl pretty right on with that? Was there anything else that you were feeling that he wasn’t able to see, maybe?
Hayley: Shock was the first emotion. I was pretty much in disbelief that it could be cancer. I also didn’t quite know what lymphoma was. When they said the word, I didn’t know that meant cancer. I asked, “Is this terminal? Is this something that can be treated?” Now I know there are over 180 forms of non-Hodgkin’s lymphoma.
I didn’t know what to think or what to expect. My doctor and I are close, so I asked, “Am I going to die?” He told me no, so that was helpful, even if he didn’t know. At least during that time, I knew it wasn’t a death sentence.
I was scared. Carl and I had only been together for a year and a half, so I had a pit in my stomach. What was I signing him and his kids up for? But he’s been good about being strong and not making me feel that anymore. I couldn’t help but think that. We’re 30 years old. When we got together, this wasn’t what he was thinking we were going to buckle up for. I had my last chemo recently, so it’s still new and there’s still fear percolating in there. It’s been a lot.
I needed to know if I had a future. I kept thinking, ‘Am I going to be able to have children? Am I going to get married? Do I tell him to go live his life and not wait for me?’
Hayley
Stephanie: I can only imagine. It was a while ago for me, but it’s still a lot coming at you and you’re still in it. Hayley, how about with Carl? You were in the room together that day. But for the days and weeks after, what did you notice about his reaction and what he was going through?
Hayley: He held it together pretty well. He was listening, very supportive, and understanding. We’re going to get through it no matter what happens. It was nice to know that he didn’t run away and wasn’t like, “I don’t want to get into this. This seems like a stressful time.” He stood by me and we’re going to get to the end of it, whatever that is going to be. Despite having two younger kids, we were going to get through it all together
It all happened so fast. After I got my diagnosis, I went to an oncologist the very next day then the day after that, I had surgery. Two weeks later, I started with treatment. I feel like my oncologist was trying to say as little as possible and not tell me much. He wanted me to focus on treatment. We’ll get there when we get there and talk about all that later. But for me, that wasn’t good enough. I needed to know if I had a future. I kept thinking, “Am I going to be able to have children? Am I going to get married? Do I tell him to go live his life and not wait for me?” That went through my head many times.
Stephanie: That hits home for me because that was where I was in my relationship, too. I thought all the same things. We imagine this life then cancer comes and it wasn’t part of the plan, obviously. Thank you for sharing that, Hayley, I appreciate it. Carl, was there anything that you feel Hayley didn’t see at the time or did she cover all of it?
Carl: No, she did a good job. None of those thoughts ever crossed my mind, but there was some underlying fear and things that I probably suppressed a little bit to be there for her. But a lot of the same emotions she was feeling, I was, too. I probably didn’t show them quite as much in the beginning.
I probably suppressed a little bit to be there for her. But a lot of the same emotions she was feeling, I was, too. I probably didn’t show them quite as much in the beginning.
Carl
Processing Your Emotions as a Care Partner
Stephanie: It sounds like you were feeling all those emotions but in order to support Hayley, you didn’t want to access them right away. Have you been able to process it more since then?
Carl: I feel like I have. We’ve been submerged in this for six months now. I’ve had my moments.
Stephanie: Can you talk about what those moments look like for you?
Carl: My first big one was when she went in for her biopsy. I stayed back while her parents went in to see her off. It was tough to see her go back. We’ve had a couple of difficult moments together around the house and some of the scary times where when she got sick during treatment, but my biggest one was while she was in surgery for her biopsy.
Stephanie: Andrew, how about you? Were you able to process early on, or did it also take some time? Do you remember the first time it hit you emotionally?
Andrew: It was when Milissa went up for her first treatments at Memorial Sloan Kettering. She didn’t feel well at all after her first treatment, so it worried me. She had to go to the ER afterwards. That’s when it emotionally hit home for me that this is very serious. We don’t know what the outcome is going to be and it’s going to be a fight.

Major Challenges Faced by Care Partners
Stephanie: Craig, what was the most challenging part of the role of care partner?
Craig: The hardest was when she was having treatment, seeing the wasting away of her body and what the drugs were doing to her. She went from a fit and healthy young lady to skinny and weak. It was tough. You’re looking from the outside in and it’s very difficult to see your partner go through that change. I said to her a thousand times that I’d swap places in an instant, but unfortunately, that’s not how it works.
Stephanie: Andrew, when we were chatting before, Milissa mentioned that she has been a nurse for over 30 years, so being the patient was a hard transition. Milissa, you called yourself a “bad patient.” Andrew, was that challenging for you? What was that like for you?
Milissa researching morphed into finding other people who were in the same situation. It became a support group for her, too, which was a good thing and continues to be a good thing for her.
Andrew
Andrew: It was very challenging. Milissa wanted to do a lot of research and that’s her nature. She would say that would help ease her mind, but I felt differently. I tried to accept that, but I felt that it would make her more anxious. I wanted her to try to put those things aside and still live her life, which is maybe a naïve way of looking at it. I couldn’t do much because I don’t have a medical background and have a limited understanding of the technical aspects of things.
In the end, Milissa researching morphed into finding other people who were in the same situation. It became a support group for her, too, which was a good thing and continues to be a good thing for her. She has taken that support group and given back to people who are going through the same experience with lymphoma. It’s been great for her because it helps her heal a lot.
Stephanie: What you’re speaking to, I’ve heard many times over. No matter how great the relationship is, there is something to be said about each person’s role and the understanding of what it feels like, which is why I’m glad we’re having this discussion with all of these perspectives.
I had what I thought was quite an unusual treatment because I haven’t met anybody else who had the same treatment as I did.
Nicky
Deciding on the Right Follicular Lymphoma Treatment
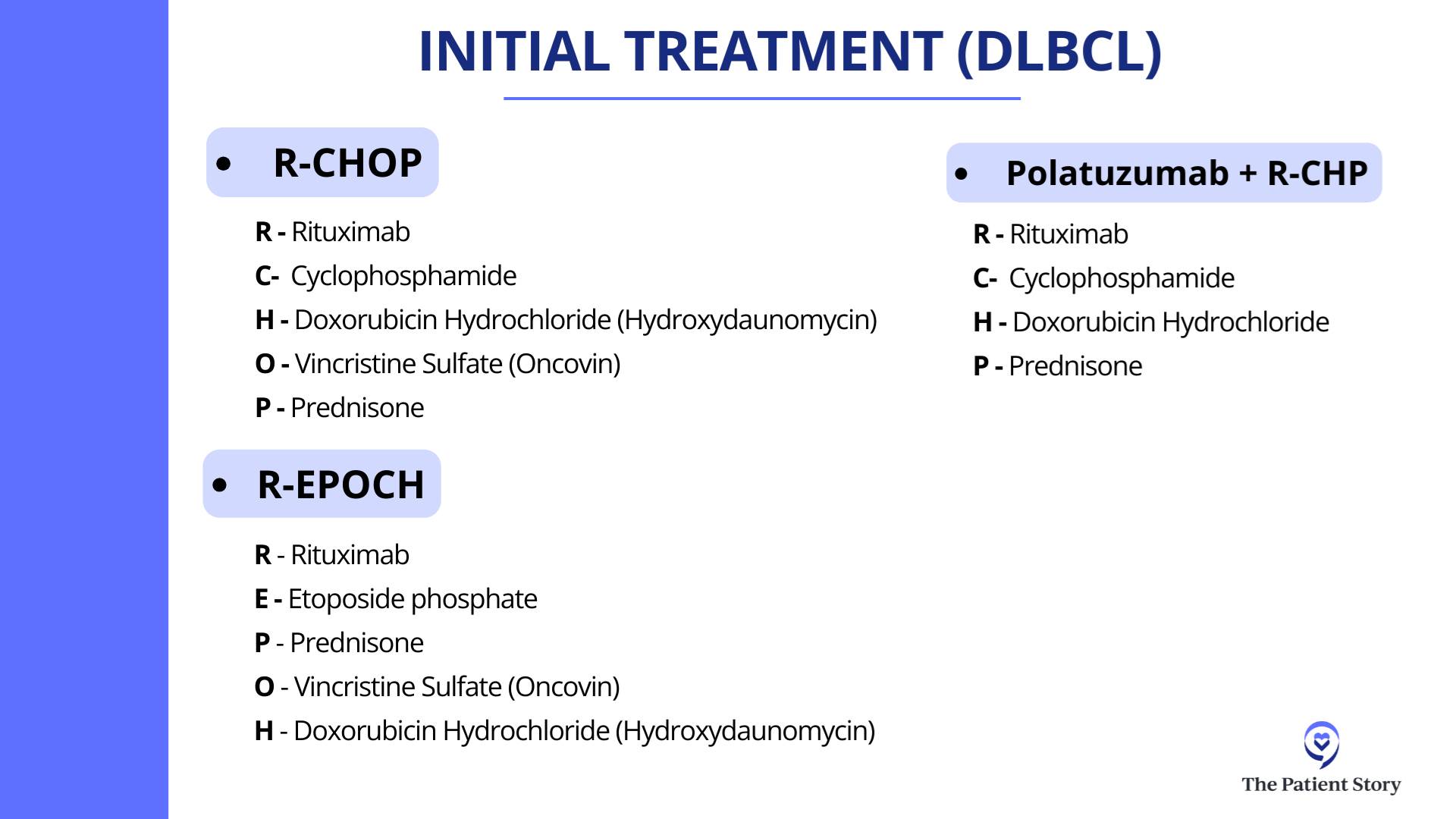
Stephanie: Could everyone share what treatment they’ve had and what that looked like, and then shift into the treatment decision-making conversation? Hayley, what treatment have you had and what’s ahead?
Hayley: I had R-CHOP chemotherapy and then Neulasta (pegfilgrastim). I’m doing at-home Neupogen (filgrastim) injections for 10 days to boost my white blood count. I took my last prednisone two days ago. I have a PET scan and labs in six weeks, but as far as meds go, I don’t have any more, nor do I want any more.
Stephanie: I hear you. Watch and wait, or active surveillance, is a big part of follicular lymphoma. Milissa, what have you had in terms of treatment?
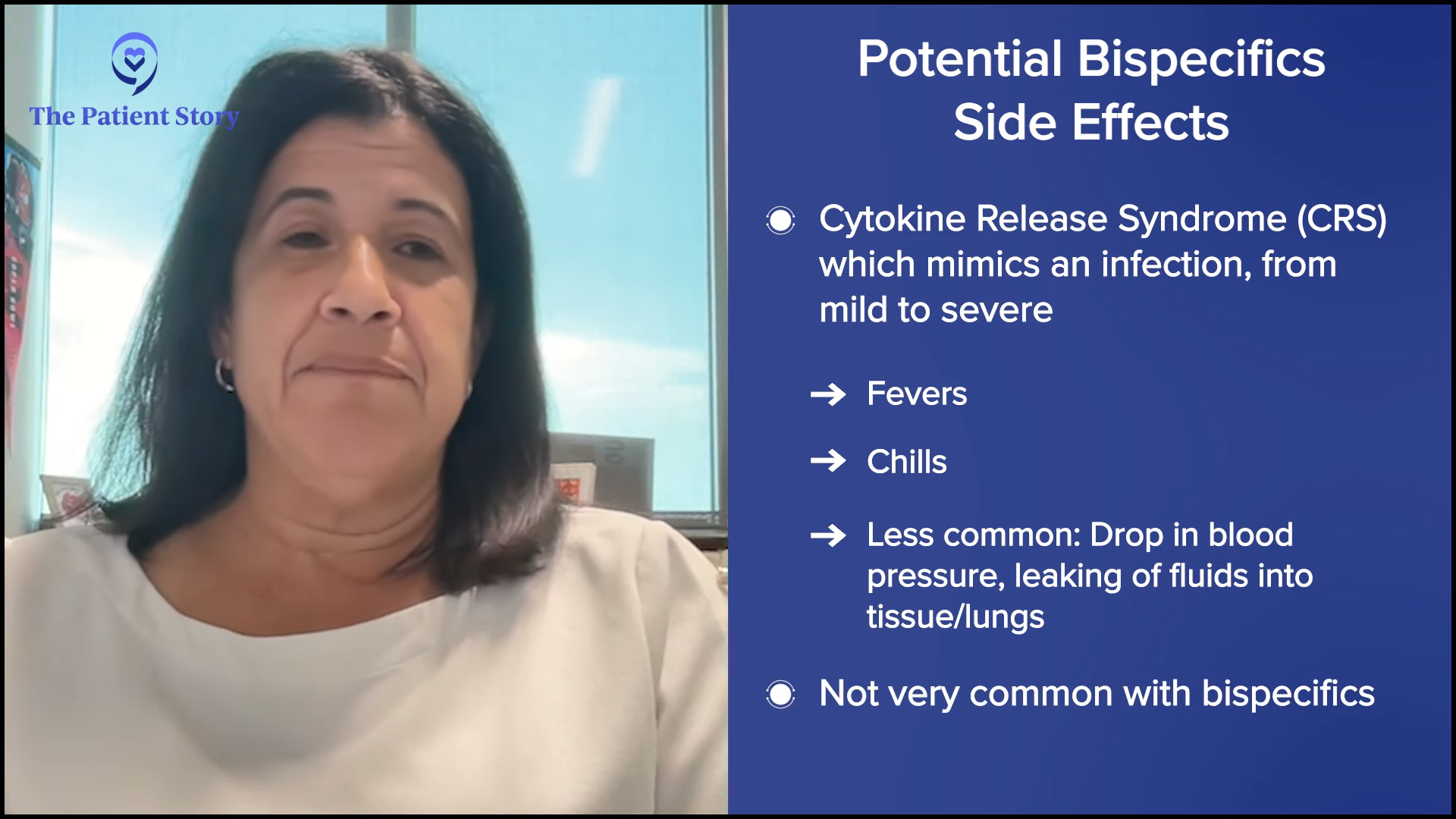
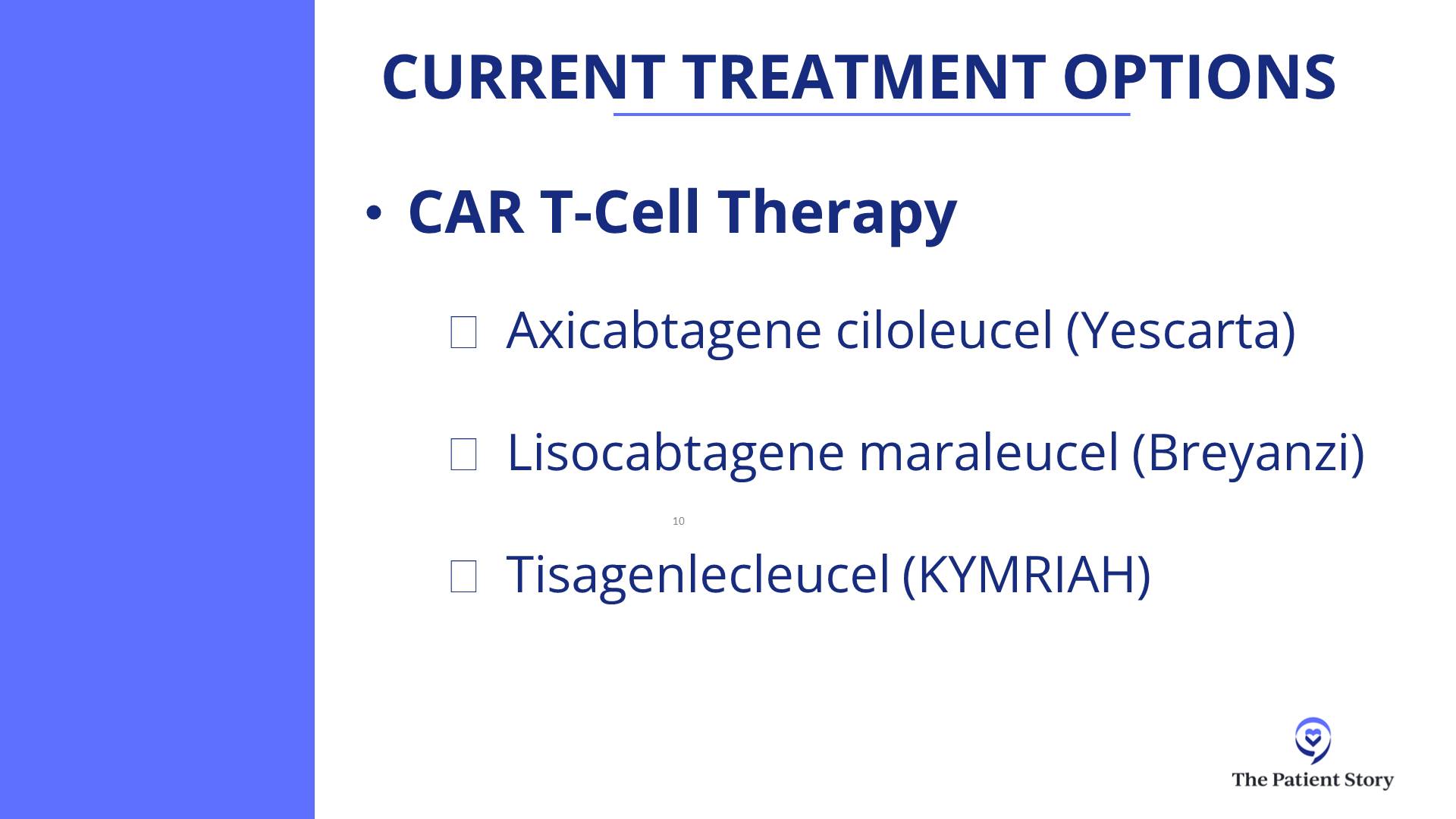
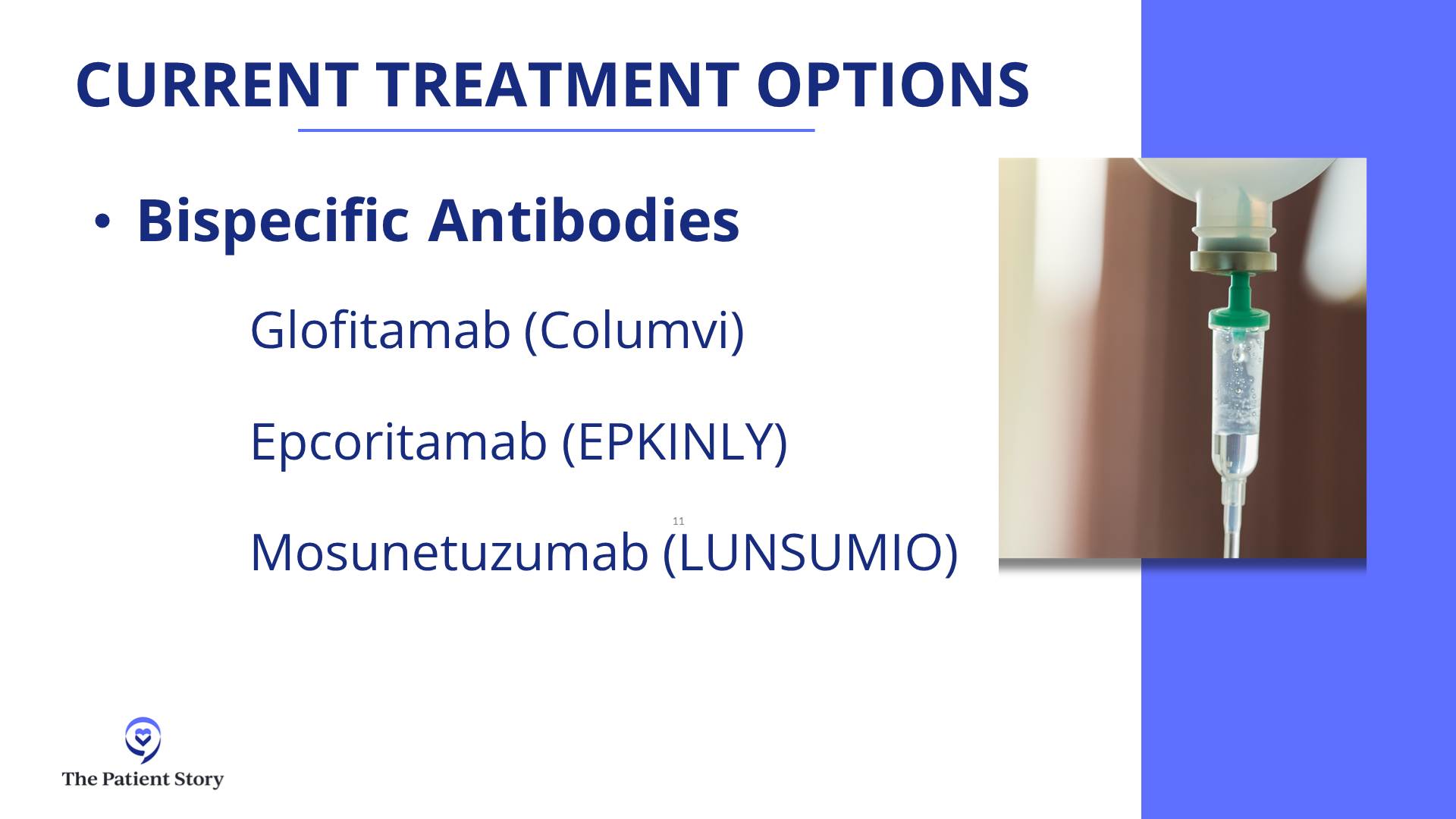
Milissa: I had a research drug called mosunetuzumab, a bispecific antibody, which was a good decision. I had eight rounds of that.
Stephanie: Were you on that after some period of watch and wait?
Milissa: No. I was diagnosed in December 2023 and started treatment in January 2024.
Because of the radioactive iodine, I’m trying to stay away from radiation as much as I can.
Nicky
Nicky: I had what I thought was quite an unusual treatment because I haven’t met anybody else who had the same treatment as I did — and I know a lot of FL patients. They put me straight into a clinical trial, so we didn’t have decisions to make, but I wasn’t educated enough to know what I was going to get into.
They suggested I go on a clinical trial of rituximab coupled with radioactive iodine. I was radioactive and put under house arrest for a couple of weeks. I wasn’t allowed within five meters of anyone or 100 meters of children or pregnant women. That was quite confronting, being away from the kids and the whole family for a couple of weeks.
After the infusion of radioactive iodine followed a year’s worth of rituximab and it worked pretty quickly. The lumps melted away within a month or two. Three months into my treatment, I had a PET scan and it was clear. I was no evidence of disease (NED).
I haven’t had a scan since then. Because of the radioactive iodine, I’m trying to stay away from radiation as much as I can. I do have a couple of lumps in my neck that we monitor, but I haven’t needed any treatments since. Fingers crossed that I don’t need any more. But the treatment fractured my immune system, so I manage that, but I manage it pretty well.

Stephanie: I’m glad to hear it. That was a good background to understand if you had these long periods of waiting. To open this into a broader discussion about communication with the healthcare team, you have two people in this: the patient and the care partner. First, there’s the treatment decision-making part, if you had that conversation with your doctor, and then symptom management and reporting what people are feeling. Hayley and Carl, how did you feel you took on the roles when it came to making decisions about your treatment?
Hayley: As far as medical treatment decisions, what do you think, babe?
Carl: I stay in my lane. I stay quiet. I take notes so she doesn’t forget what the doctors say.
Stephanie: Taking notes is good because a lot is coming at you all at once.
I was looking for any glimmer of hope, even if it came with 50 bad things that I read. If I could find two supportive statements that made me feel better, that would be helpful.
Hayley
Hayley: When Andrew was talking about Milissa researching and he was thinking it would make her more anxious than not, I was trying to figure out where that was coming from because I did the same thing. I was looking for any glimmer of hope, even if it came with 50 bad things that I read. If I could find two supportive statements that made me feel better, that would be helpful. But with treatment, I made a lot of those decisions, but they didn’t give us any options because it had transformed.
Carl: Put a lot of trust in the doctors, too. We ask questions, even retrospectively, on why this and not this, and they had very detailed answers to everything. At the time, it was happening so fast. As she said, she didn’t necessarily have a choice, but it all worked out.
Stephanie: You said you stayed in your lane, which I think resonates with a lot of people. I wonder how you were able to do that because, of course, you care for Hayley, so you want to do something. Did you channel wanting to help in a different way, outside of taking notes? How did that manifest for you?
Carl: I helped with the day-to-day stuff as much as I could. She’s pretty strong-willed and independent, so I try not to make her feel like she’s being babied too much. We cleaned up the food around the house and did the typical things hygiene-wise to try and stay healthy and support her.
It was only a year and a half into knowing them, so the emotional connection was different because I couldn’t be as affectionate.
Hayley
Hayley: That was rough. He has an 11-year-old daughter and a six-year-old son. They were both a year younger when I got diagnosed, but I’m close with the 11-year-old girl. We’re buddies. It was sad because I knew that she would probably grasp a little bit. After all, her friend’s mom went through leukemia and it scared her badly. She started crying. She was scared for me. We try to let her know that it’s going to be okay without completely lying and saying I just have a cold, so that was a teeter-totter for sure.
With the younger boy, we try not to make him feel like he’s doing something wrong by continuously reminding him to wash his hands, change his clothes, or shower. Because all of a sudden, I’m like this bubble and he has to stay away from me when we used to lie on the couch together. “Does she not like me anymore? Why is she not being so lovey?” It was only a year and a half into knowing them, so the emotional connection was different because I couldn’t be as affectionate. I was stressed and, to be honest, I thought of them as little germ factories when they came home and I was scared. After I got diagnosed, I went into neutropenic fever and stayed in the hospital for a week. It was a lot to endure.
When they got a little bit older, we had a conversation with them. The emphasis was that when you hear the word ‘cancer,’ it’s not something to be immediately scared of.
Craig
Stephanie: You’ve gone through a lot, but I appreciate you bringing this up because there’s the obvious stuff and then there are all the other things that come with treatment that impact day-to-day life. Nicky and Craig, with the kids, did you play different roles in communicating the diagnosis?
Nicky: We didn’t tell them.
Craig: The kids were too young when Nicky was going through treatment. When they got a little bit older, we had a conversation with them. The emphasis was that when you hear the word “cancer,” it’s not something to be immediately scared of. There is a lot of information. There are different types and a lot of different scenarios. We wanted to reassure them that mum has a condition, but it’s something that we manage and something that we’re going to manage for the rest of her life, so that’s how we move forward with that.
Nicky: Our daughter was five when I finished treatment. When I went away, I told her it was for work and she was mad at me for going away. She noticed how it affected my mental health. For the whole year after finishing treatment, before I set up the Facebook group, I was absolutely miserable and she felt that. It wasn’t until I realized how much she felt that was when I knew I had to do something about my mental health.
Our youngest was very little. He wasn’t even one when I was diagnosed, but when we told them, like Craig said, we didn’t want them to be scared of the word cancer. We decided to tell them when we were doing some fundraising for the leukemia foundation. It was a lantern night, so we had fun. We explained why we were fundraising and why mum has a white lantern and everyone in the family has a blue lantern. We try to explain it in a way that wasn’t scary for them.
I didn’t know much about the clinical trial drug because it hadn’t been out very long, maybe two years, so there wasn’t much information, which was scary.
Milissa
How Care Partners Can Help with Treatment Decision-Making?
Stephanie: Milissa and Andrew, what were the roles that you played in treatment decision-making in the doctor’s office? Did you find that one was speaking up more than the other?
Milissa: I have a mini homestead, so I have several animals to take care of, so thankfully, he stayed back for that. I went by myself a couple of times and then my mom went with me for the decision-making. I was given two options for treatment: one was the clinical trial and the other was rituximab and Revlimid (lenalidomide). The doctor waited for the clinical trial. He pushed me into it creatively.
Stephanie: Can you talk about that more? What did he say and what resonated so that you decided to go in that direction?
Milissa: I, of course, researched both. I didn’t know much about the clinical trial drug because it hadn’t been out very long, maybe two years, so there wasn’t much information, which was scary. It seemed like my insurance would pay more for the standard treatment, so I was leaning more toward that.
My doctor told me that he would have the pharmacist talk to me about the standard treatment; the clinical trial nurse talked to me about the clinical trial. I’m glad I did it. He said that the clinical trial had fewer side effects and discussed those with me at length. He also discussed some of the side effects of the others. That’s when I decided to do the bispecific antibody.
From very early on, she knew that was the route she wanted to go, so that fell into place very quickly.
Andrew
Stephanie: Andrew, when Milissa is talking to you about these treatment options, did you agree? What thoughts did you have about the decision?
Andrew: I was a little concerned about the clinical trial, like how much is known about it and the success rate, but Milissa was concerned about the side effects of the more traditional treatments. If I remember, the insurance would cover her to receive these clinical trials at Memorial Sloan Kettering Cancer Center in New York City. I don’t know if she would have been able to receive that locally, but we had some unsatisfactory experiences with some of the local healthcare facilities. Memorial Sloan Kettering is world-renowned.
My only concern was her going to New York City by herself because she couldn’t go on public transportation or fly, so she needed somebody to take her. Fortunately, her mom stepped up and would take her to New York and I would stay behind and take care of the farm.
Stephanie: How important was it to have Milissa’s mom? Who else was part of the regular support circle and how important was that?
Andrew: Her mom was supportive. Sometimes she would get stressed with her mom because her mom had her thoughts about things and that’s normal. I know she would confide in some of her girlfriends and seek support from them as well. But from very early on, she knew that was the route she wanted to go, so that fell into place very quickly.

Stephanie: Hayley and Carl, and Nicky and Craig, were there additional major players in your support circle?
Hayley: Definitely both sides of our family, but I would say the next after Carl is my mom. She definitely has been there every step of the way. The only time she wasn’t was when she was sick and when my dad got sick. She was in tears over not being able to be at treatment with me. She definitely made it very clear that it doesn’t matter what’s happening or what time of day — she wants to be there and be able to help me.
We’re similar in some ways and opposite in a lot of ways. We butt heads at times, but she put everything to the side. There are things about her that drive me crazy and she can be a little overbearing, but whenever she’s at the doctor, she’s able to turn it off and let me do my thing.
One time, she begged to be in the room to see the doctor and I remember saying, “I have a lot of questions. It’s already a small room. We have the doctor, the nurse coordinator, me, and him (referring to Craig). There’s no room for you in there.” She asked the people at the front desk, even though I said no, and still came and knocked on the door. I let her in and said, “I have a lot of questions for the doctor and a lot of stuff I want to cover, so I need you to reel it in,” and she did. She sat there quietly and was grateful to be in there. But she’s been good throughout the whole thing.
She definitely made it very clear that it doesn’t matter what’s happening or what time of day — she wants to be there and be able to help me.
Hayley
Stephanie: It sounds like you were very clear with your communication in setting boundaries.
Hayley: Yeah. They needed to be set.
Carl: It’s one of Hayley’s strong suits.
Hayley: And she did a good job.
Carl: The web runs pretty wide with us. My parents were great in helping out with the kids. I have them 50% of the time, so we aligned our schedule with chemo. The first one got delayed, so all bets were off after that. We had to be fluid and flexible. We had help from both sides of the family all the way down the list at some point, whether it was with the dogs, the kids, or everything in between. It was pretty great. A lot of friends reached out. She received a lot of care packages and flowers. Those meant a lot in those times.
Hayley: You don’t realize how much they mean. Flowers have never meant more. They showed up at the door and my whole day became better. I wish that I didn’t know how people could make someone feel, but now I know moving forward for not if but when I know somebody that goes through this. I’m almost thankful that I know how to take care of them and I’m excited to pass along what people have done for me.
We had great support on both sides of the family… the people I am friends with are like family… Whatever I or Nicky needed, they were able to accommodate that for us and I’ll be forever grateful for that.
Craig
Stephanie: That’s beautiful because you’ve been in this position and now you’re paying it forward with all that empathy. Nicky and Craig, how about you? Did you have additional support and how important was it to have that?
Craig: We had great support on both sides of the family as well. We have an extra network of our friends who we’re close with; these were people we grew up with and stayed friends with. I don’t have a large circle of friends, but the people I am friends with are like family. It was good to be able to rely on them and fall on them when I needed to debrief and maybe forget about things for a while. Whatever I or Nicky needed, they were able to accommodate that for us and I’ll be forever grateful for that.
Nicky: We’re lucky that my parents live about 200 meters down the road from where we live. Although at the time of my diagnosis, my mum wasn’t well, so she couldn’t be there for me. But Craig’s mum was a pillar of strength, particularly for me. She lives about an hour away from us, but she took me to my first treatment. I stayed at her place because I had my treatment in Perth. She has metastatic breast cancer. At the time, it wasn’t metastasized, but she had been through breast cancer, so we connected on that level as well. She was probably one of our biggest supporters, as well as my parents and our close friends. We’re very lucky because when I went away for a couple of weeks, we had family support the kids so Craig could keep working and I was appreciative of that.
We got used to operating on a little bit of a higher stress level with dealing with treatments and the whole situation.
Carl
Biggest Emotional Challenges for Couples
Stephanie: For Hayley, Carl, Milissa, and Andrew, did anything resonate for you in terms of the emotions and what you found was the most challenging? Have you been able to talk about it with each other or did you talk to other people about it and preserve your relationship differently?
Carl: It was always the outside stresses that caused the most tension, like one of the kids getting sick or something like that. Going through treatments, Hayley was very lucky. She definitely had some bad moments, but she had more good days than bad days. I would think that would be fair to say. We still had a lot of normal, if you will, quality time together, even through treatments. But when something would go wrong, unforeseen challenges piled on top of everything else.
We got used to operating on a little bit of a higher stress level with dealing with treatments and the whole situation. We were already at our tipping point, so at times when something else would get introduced, it would put us over the edge, but we always got to settle down and bring it back. What helped us was being grounded and centered at home. Honestly, it’s one of the things that I see as a benefit and a net gain out of this. We got a lot of quality time in our house. All the running gets cut out. You trim back the activities, which can be hard but at the same time, it was definitely good for us and the kids.
I was too focused on making sure that Milissa was staying positive and trying to take care of things… I have my little hobbies and things that I do on the side to help ease my mind.
Andrew
Stephanie: There’s always attention on the patient but maybe a lot less so on the care partner — that was my experience at least. Everyone was worried about me. I remember telling my now husband, “Hey, what you need, you need. Make sure to take care of yourself.” How did that show up for you, Carl, or did it not?
Carl: It did in ways. I have some property about an hour away, so when it would build up, I’d escape there for a few hours and take time when I needed it. Luckily, I didn’t need a whole lot, but Hayley was always understanding of it. She’d be able to tell. We’re pretty in tune with each other, so it ended up working out okay on my end.
Stephanie: Andrew, how did that show up for you? Did you take care of yourself? Was it a thought that crossed your mind?
Andrew: No, I was too focused on making sure that Milissa was staying positive and trying to take care of things. I went with her a couple of times for her treatments, so it was comforting for me to be able to accompany her, feel what she was going through, and see how the process was. It eased my mind a lot. I felt that she was getting good care there, so that made me feel good. I’m the kind of person who stays busy and that helps. I have my little hobbies and things that I do on the side to help ease my mind.

Biggest Lessons Learned as a Patient and Caregiver
Stephanie: You shift from being partners in one way to this completely different dynamic of patient-care partner, so things like emotional health, mental health, intimacy, and the way that you look at one another in all these different ways can be impacted. I think that’s very natural. What is the biggest way that you saw that impacted the relationship and the biggest solution, too?
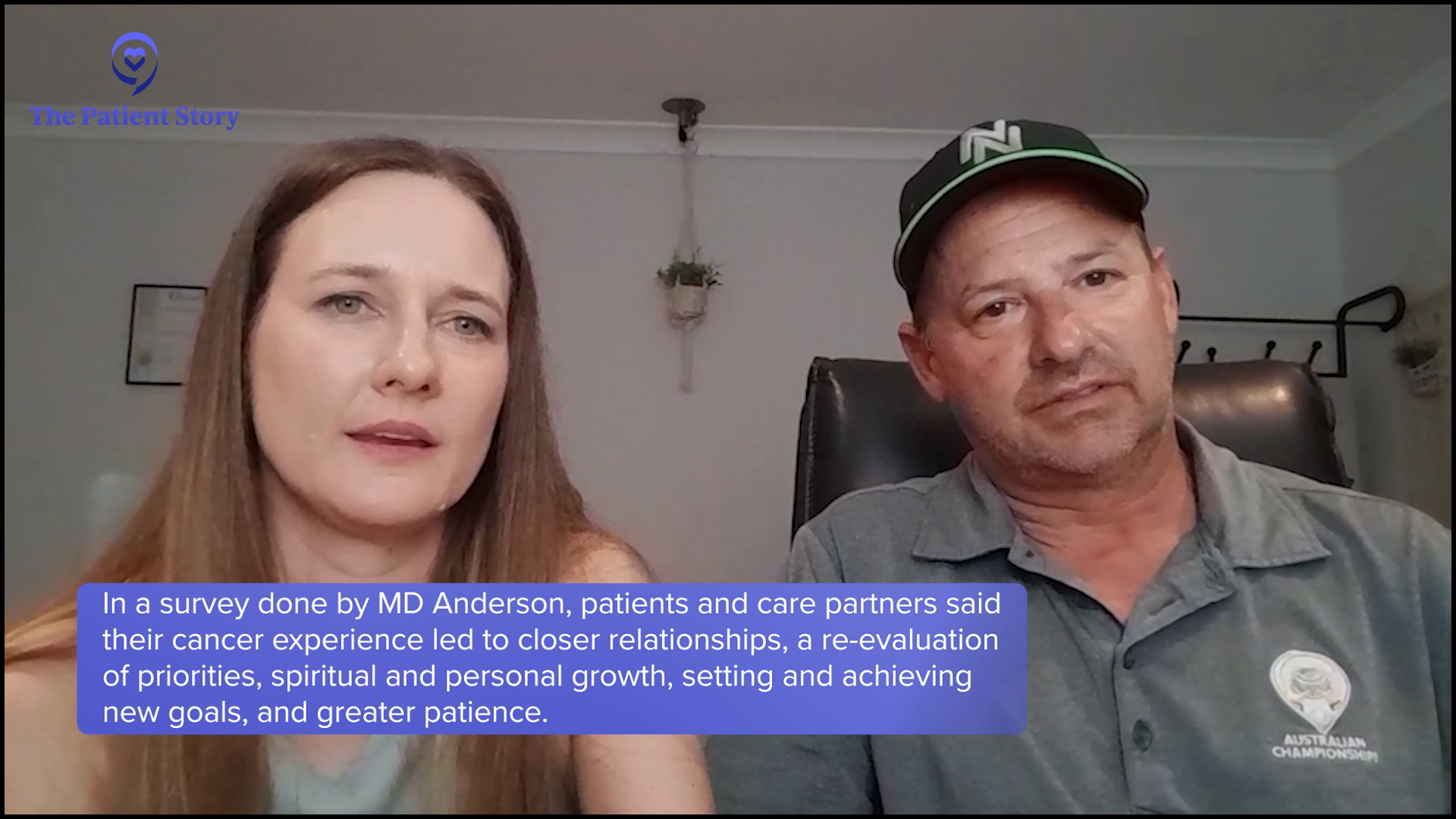
Nicky: It definitely brought us closer together, didn’t it?
It makes you realize how fragile life is… We’re more thoughtful with how we approach everyday things. You don’t sweat the small stuff anymore because it’s not worth it.
Craig
Craig: Yeah. It definitely made us a lot stronger and more resilient, and changed our perspective on everything. We definitely take a lot more care with the kids and make sure we soak up all the little moments. It makes you realize how fragile life is. To try and put a good spin on this, our lives changed dramatically with the diagnosis, but for the most part, our lives have changed for the better.
Nicky: Absolutely.
Craig: We take better care of ourselves. We’re more thoughtful with how we approach everyday things. You don’t sweat the small stuff anymore because it’s not worth it. Those insignificant things don’t matter because when you think about the big picture, what you’re trying to achieve, and the direction you’re heading and where you’re going, you don’t have time for the small stuff anymore. It’s the big stuff.

Nicky: The mental health aspect was probably the hardest for me. He was an incredible support for me during that time. As I was leaving the hospital, I remember them saying, “Expect it to come back in three to five years, but if it comes back in two years, your prognosis is worse,” so that stayed with me. When I realized I needed to do something about this, I felt like the only thing that would help me at the time was to connect with other people with the same diagnosis. At that time, we couldn’t find anybody. Craig suggested, “Why don’t you start a Facebook group and let them come to you?” That’s exactly what I did and they did. Instantly, all that stress just melted and I felt like I found my people.
Stephanie: Milissa and Andrew, what was the biggest shift for you and the biggest piece of guidance you have for others on that?
Milissa: It was finding people who have gone through the same thing. I joined another research study at Colorado State University where they did exercise afterwards and that changed my life. Now I lead a group that exercise online afterwards and we still stay together. It’s a national group, which is awesome. But for us relationally, it’s evolved. I feel like in the relationship, we’re in the infantile stages and we learned about each other, what our strengths are, and what each other can give, so that was a good thing.
Andrew: The diagnosis and going through the experience of treatment made our relationship evolve. We became a lot more emotionally mature as a couple. It’s still a growing process because you still have to maintain your care and your treatment, but we’re in a much stronger place than we were when we first got the diagnosis.
We learned about each other, what our strengths are, and what each other can give.
Milissa
Stephanie: Hayley and Carl, I know this happened not too far into your relationship and it was a lot to go through. What guidance do you have for other people?
Hayley: I feel like it pushed issues that we hadn’t even come across yet because it was only a year and a half into our relationship, so we had to learn a lot about how we dealt with things. I had never seen him be emotional. I am not an emotional person and then all of a sudden, we’re a wreck for a while and let all of our walls down at one time. It seemed like it would have taken 10 years normally to go through what we went through in 90 days. It escalated our communication and understanding a lot quicker than it normally would have in a relationship.
I know that follicular lymphoma will come back, but I’m ready and I’ve come to terms with that… I’m not nearly as scared as I was at the beginning.
Hayley
But I do think that some positives come of it in general. I can appreciate life more. The meaning of everything is a lot different and I feel like people don’t realize that they treat life as a disposable item, but they do. I’m more appreciative. Looking outside at the green grass seems different from last year. I can’t wait to sit on the beach. I told Carl, “I’m probably going to start crying sitting on the beach this year because I’m so grateful to be here,” especially whenever you think about the alternative that goes through cancer patients’ minds. It’s also new for me, so I’m excited to be on the other side right now.
I know that follicular lymphoma will come back, but I’m ready and I’ve come to terms with that. I’m okay with it being something like a condition, like Nicky and Craig said. It’s going to come back, but there are so many treatments for it. I feel like it’s moving at such a fast rate that I’m not nearly as scared as I was at the beginning.
Carl: I would say quality time was huge for us in terms of what helped us. Within that is obviously talking a lot, whether it was about cancer or not. While hanging out on the couch or in bed, I tried to make a point to get away from cancer talk sometimes. We’re goal-oriented, so we talk about short-term goals and long-term goals, reminding ourselves that there’s another side to this, which we’re starting to see now.

Conclusion
Stephanie: Thank you all for an insightful conversation. Because you’re all at different points of this experience, I think this discussion is going to help so many people who are experiencing different situations. It was so great to hear your perspective in-depth, so thank you so much for joining us.
Thank you again to our sponsor, Genmab, for its support of our independent patient program, again allowing us to be able to host more of these for you. The Patient Story always retains full editorial control.
We want to point out again some of our incredible resources from our friends at The Leukemia & Lymphoma Society and the Living with Follicular Lymphoma Facebook group. The LLS has a community section for people to meet and chat with other blood cancer patients and care partners. It also has free personal guidance through Information Specialists. The Living with FL Facebook group features an ongoing lively chat in real-time with thousands of people from across the globe. It is the biggest FL-dedicated Facebook group that there is.
We hope that this was helpful for you. Thank you so much and we hope to see you at another program soon. Thank you and take good care.
Thanks to The Leukemia & Lymphoma Society and the Living with Follicular Lymphoma Facebook Group for their partnerships.

Thank you to Genmab for supporting our independent patient education content. The Patient Story retains full editorial control.
Non-Hodgkin Lymphoma Programs
Follicular Lymphoma Patient Stories
Hayley H., Follicular Lymphoma, Stage 3B
Symptoms: Intermittent feeling of pressure above clavicle, appearance of lumps on the neck, mild wheeze when breathing and seated in a certain position
Treatments: Surgery, chemotherapy
Laurie A., Follicular Non-Hodgkin Lymphoma, Stage 4
Symptoms: Frequent sinus infections, dry right eye, fatigue, lump in abdomen
Treatments: Chemotherapy, targeted therapy, radioimmunotherapy
Courtney L., Follicular Lymphoma, Stage 3B
Symptoms: Intermittent back pain, sinus issues, hearing loss, swollen lymph node in neck, difficulty breathing
Treatment: Chemotherapy
John S., Follicular Lymphoma, Stage 4
Symptom: Swollen lymph nodes
Treatments: Clinical trial, chemotherapy