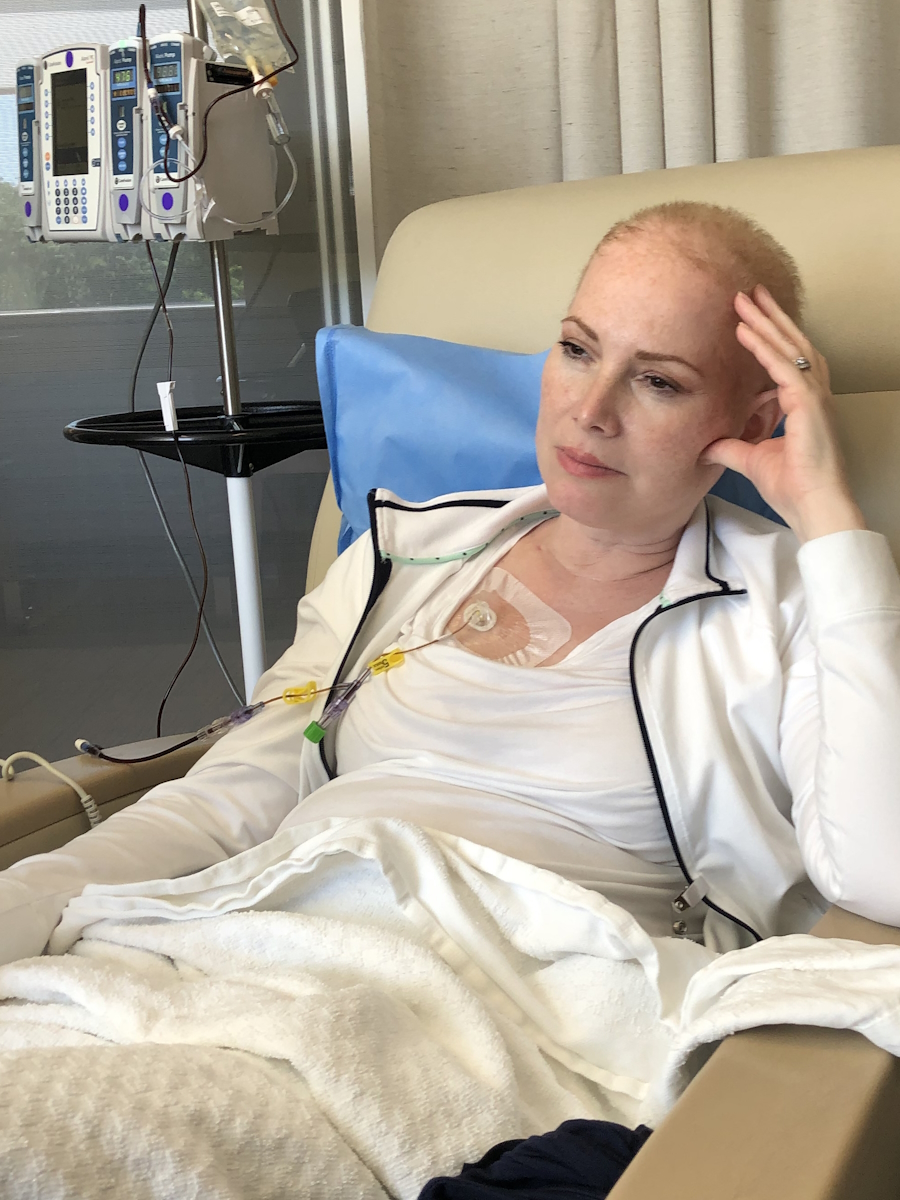
40 Years Living with an MPN: Jeremy’s PV and Myelofibrosis Story
Living with polycythemia vera (PV) has been a lifelong balancing act, and both Jeremy Smith and MPN expert, Dr. Angela Fleischman from the UC Irvine Chao Family Comprehensive Cancer Center bring wisdom, encouragement, and practical advice to the conversation.
Interviewed by: Taylor Scheib and Stephanie Chuang
Edited by: Katrina Villareal and Jeff Forslund
Jeremy has been living with an MPN for nearly four decades, initially being diagnosed with polycythemia vera (PV) that progressed to myelofibrosis in 2012, and he is refreshingly honest about the emotional and physical challenges he’s faced along the way. His diagnosis started with what felt like a panic attack, leading to an emergency room (ER) visit and eventually a bone marrow biopsy. Like many people, he found the process confusing and overwhelming.

Today, Jeremy takes charge of his care. He calls his medical team his “board of advisors,” reminding other patients that they are the ones who ultimately make the decisions. He’s tried multiple treatments and is currently taking interferon alpha as part of a combination therapy, which works well for him despite not being a standard regimen. He stresses the value of second opinions, open communication with doctors, and writing down questions before appointments. Jeremy also highlights exercise as one of the most powerful tools he’s found for immune health and inflammation control. He even transitioned from mountain and road biking to riding a stationary bike in order to protect both his spleen and his ability to still ride.
Dr. Angela Fleischman, MD, PhD, a hematologist and passionate MPN researcher with the UC Irvine Chao Family Comprehensive Cancer Center, adds an equally empowering perspective. She focuses on patient-centered care, encouraging people with MPNs to understand their disease and actively participate in decisions. She believes that knowledge is a powerful tool and that patients should feel comfortable asking questions, seeking second opinions, and exploring clinical trials.
Together, Jeremy and Dr. Fleischman paint a hopeful picture. While polycythemia vera is complex and often unpredictable, staying engaged in care, building a team you trust, and prioritizing overall health can make a huge difference. Jeremy’s parting advice says it all: take notes, ask questions, connect with others, and embrace life one moment at a time.
Key Story Takeaways
- Jeremy’s story: Living with polycythemia vera (PV) for nearly 40 years, Jeremy shares how his disease progressed to myelofibrosis (MF) and how he manages treatment, side effects, and mental health.
- Treatment insights: He uses interferon alpha in combination with another drug (two separate medicines) while emphasizing second opinions, symptom tracking, and exercise to reduce inflammation.
- Expert perspective: Dr. Angela G. Fleischman, MD, PhD of UC Irvine explains PV biology, the role of interferon alpha, and how patient-doctor partnerships and personalized care improve day-to-day living.
- Empowerment message: Stay informed, build a trusted medical team, keep notes and questions ready, and live fully—sip life—despite PV or MF.
- Name: Jeremy S.
- Age at Diagnosis:
- 33
- Diagnoses:
- Polycythemia Vera (PV) that progressed to Myelofibrosis (MF) in 2012
- Chronic Lymphocytic Leukemia (CLL)
- Treatment:
- Interferon alpha in combination with another therapy


Thank you to PharmaEssentia for supporting our independent patient education program. The Patient Story retains full editorial control over all content.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make informed treatment decisions.
The views and opinions expressed in this interview do not necessarily reflect those of The Patient Story.
- Jeremy’s PV Story
- Dr. Angela Fleischman
- Introducing Dr. Angela Fleischman
- Polycythemia Vera Symptoms
- How PV Patients Get Diagnosed
- Discussing Treatment Options and Patient Preferences
- Tracking Symptoms Effectively in PV
- Communicating with PV Specialists: Tips for Patients and Caregivers
- Hopes for the Future: The PV Treatment Landscape
- More Polycythemia Vera Stories
Never give up. There will be highs and lows, but you have to keep going and enjoy every moment. Sip life — embrace it and never give up.
Jeremy S. – Polycythemia Vera & Myelofibrosis Patient
Jeremy’s PV Story
Introducing Jeremy
My Polycythemia Vera and Myelofibrosis Patient Experience
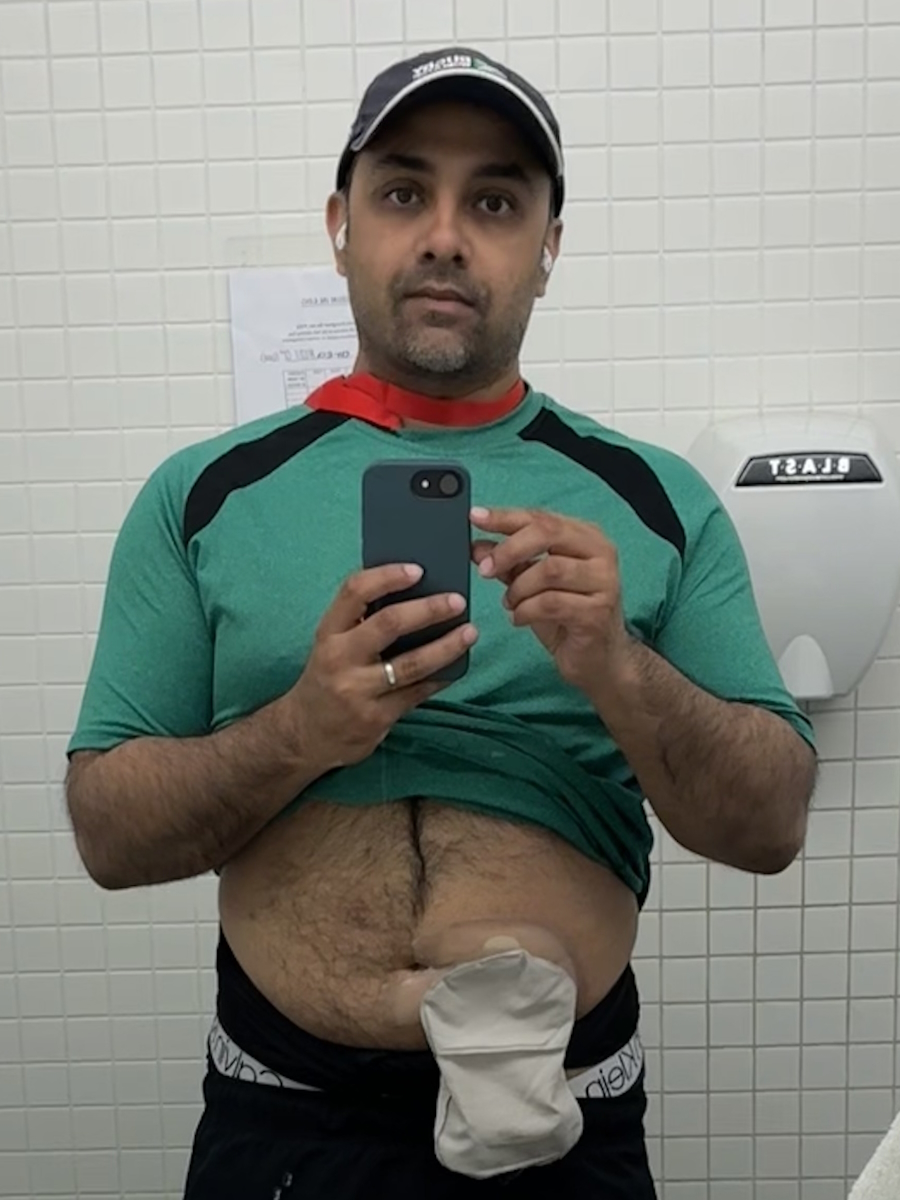
Jeremy Smith: I was diagnosed with polycythemia vera in 1989, but they think I had it for five to eight years before that. September 2025 marks the beginning of my 37th year with polycythemia vera (PV). In 2012, [it progressed] to myelofibrosis (MF). I also have chronic lymphocytic leukemia (CLL).


How My Friends Would Describe Me
Jeremy: It depends on whom [you] ask. I would say determined. Crazy, maybe. Most people did not expect me to be around this long, including my doctors. [How] we see ourselves is often very different than how others see us and, of course, they bring their own baggage to their perspective. My best friend [is] my brother, who probably has the best understanding of me since we shared a room as kids. I would also say creative; I have done a lot of creative things in my life.
You have to look at the big picture… Every option should be considered.
Jeremy S. – Polycythemia Vera & Myelofibrosis Patient
First Symptoms and PV Diagnosis
Jeremy: Like most patients, I was originally seen by a local hematologist and my primary care physician. My symptoms masqueraded as anxiety because my hemoglobin was so high. I had what I thought was a panic attack while driving home, [so I] called 911 and ended up in the emergency room. Ultimately, a bone marrow biopsy confirmed polycythemia vera. The process was confusing and emotionally overwhelming.


Navigating Treatment Decisions
Jeremy: You have to look at the big picture. Combo drugs, often referred to as cocktails, can be the most effective way forward for many patients. We do not know what drug will work best for each person until [they] try it. I am currently on interferon alpha and [another treatment] together, which has been very effective for me, even though it was not previously tested. Every option should be considered.
You must be your own advocate and write down your questions before you go to the doctor so that you’re well prepared.
Jeremy S. – Polycythemia Vera & Myelofibrosis Patient
Adapting to New Specialists and Building Trust
Jeremy: Losing a trusted doctor was devastating. Dr. Schreier was very old school, but Dr. David Iberri, who took my case at Stanford, was open, younger, and more willing to embrace new treatments. Building trust takes time, especially when your numbers fluctuate. I manage my care directly, just as I would a business, and have assembled a team of doctors who consult with each other on my medical plan.


Living Well with an MPN
Dealing with a Chronic Cancer and Aging
Jeremy: It has been a long journey. The last five to six years have been the hardest, as my body [has] begun to break down. The longer you live with a myeloproliferative neoplasm (MPN), the more your body gives in structurally.
Twenty years ago, we did not know the impact of anemia on myelofibrosis patients, but now we know cardiovascular issues are a major risk — and I have cardiovascular issues now. The disease is complicated, but one thing doctors do not discuss is how grueling the mental journey is. It is taxing. I spend more time with my doctors than with anyone else.
I have established what I call my board of advisors — my doctors. I do not treat them like doctors; they are advisors. I make all the decisions.
Jeremy S. – Polycythemia Vera & Myelofibrosis Patient
Building a Medical Team: The Board of Advisors Approach
Jeremy: Because the disease is so complicated and manifests in many ways, you need to be prepared. I have established what I call my board of advisors — my doctors. I do not treat them like doctors; they are advisors. I make all the decisions.
I have four hematologists and oncologists, plus a cardiologist and other specialists. They all work together and I have learned to manage the egos involved. Getting everyone on a call can be [challenging], but it works well.


The Importance of Exercise in Managing MPN
Jeremy: Exercise is a big part of my life. Back in 2000, I was riding 30–75 miles on a bike. Now, because my spleen makes all my red blood cells and my doctors are worried about damaging it, I can only ride a stationary bike.
Exercise is truly the only proven method for improving your immune system and reducing inflammation, which is key to slowing disease progression. In the late 80s, there was no data that exercise or diet would help PV, but now we know it improves fatigue and overall health.
Exercise is truly the only proven method for improving your immune system — and reducing inflammation is key to slowing down disease progression.
Jeremy S. – Polycythemia Vera & Myelofibrosis Patient
Message of Hope
Jeremy: Never give up. There will be highs and lows, but you have to keep going and enjoy every moment. Sip life — embrace it and never give up. You’re going to die anyway, so what do you have to lose?
Seek out support groups, such as [my] Facebook group MPNLIFE, and gather multiple medical opinions. Be your own advocate. Write down your questions and, if possible, take someone to appointments to help with notes.

I value the opportunity to get to know my patients over the years or even decades… We should be partners in the patient’s health journey, and I intend to accompany them throughout it.
Dr. Angela Fleischman, MD, PhD, Hematologist and MPN Researcher
UC Irvine Chao Family Comprehensive Cancer Center
Dr. Angela Fleischman

Introducing Dr. Angela Fleischman
Stephanie Chuang, The Patient Story: Would you [please] share your motivation for working with PV patients and anything in your background that helps you relate to them?
Dr. Angela Fleischman: I have been dedicated to caring for patients with myeloproliferative neoplasms (MPNs) from the very beginning of my medical career. My enthusiasm and dedication continue to grow daily, even after being in this field for quite a long time. What truly attracts me to this particular condition is multifaceted. Number one, I value the opportunity to get to know my patients over the years or even decades.
Number two, I appreciate that patients can do a lot for themselves. Self-management and self-empowerment are very attractive concepts to me as a physician. My goal is to guide a patient to maximize their health, rather than simply prescribe a medication. We should be partners in the patient’s health journey and I intend to accompany them throughout it.
Polycythemia Vera Symptoms
Common First PV Symptoms
Stephanie: What are the common profiles of patients with PV in terms of age or symptoms when they present to you?
Dr. Fleischman: Every patient is unique, but common themes do exist among PV patients. They can present at different stages in life; while PV tends to present in older adults, there are also young people diagnosed with polycythemia vera. We should not categorize patients strictly by age or type.
In retrospect, many PV patients have experienced itching for a long time without knowing its cause; they often tried different soaps or treatments without relief. If there is one distinctive symptom, it is likely chronic itching. Fatigue is common among PV patients, but it is also prevalent in the general population, making it less useful for distinguishing PV. Itching is truly the main unifying symptom.
The exact molecules driving PV-related itching are not well defined.
Dr. Angela Fleischman, MD, PhD, Hematologist and MPN Researcher
UC Irvine Chao Family Comprehensive Cancer Center
Understanding PV-Related Itching
Stephanie: How is PV-related itching different from other dermatologic conditions, like eczema?
Dr. Fleischman: The feature that distinguishes PV-related itching is its aggressiveness after a hot shower. If you hear a patient describe sudden itching induced by heat or water, that is very common in PV. Patients often say, “I feel like I’m itching from inside; my whole body is itching from inside,” which sets it apart from localized itching, such as an itchy foot. That is not typical of PV.
The Science Behind PV Itching
Stephanie: Is there a simple way to explain the science behind why PV patients experience itching?
Dr. Fleischman: It most likely relates to increased red cell mass. When patients undergo phlebotomy to lower their hematocrit, the itching often resolves. It also likely involves abnormal cytokines or inflammatory proteins produced by immune cells, although the exact molecules driving PV-related itching are not well defined.
While these are characteristic abnormalities in polycythemia vera, having a high hemoglobin or hematocrit alone does not mean a person has PV.
Dr. Angela Fleischman, MD, PhD, Hematologist and MPN Researcher
UC Irvine Chao Family Comprehensive Cancer Center
How PV Patients Get Diagnosed
Stephanie: How do PV patients get diagnosed? What do they notice and what leads to diagnosis?
Dr. Fleischman: Diagnosis occurs in many ways. Often, a patient visits their primary care physician for itching or another issue, or during a routine checkup. Abnormal labs — such as high hemoglobin, hematocrit, white count, and platelet count — raise suspicion. While these are characteristic abnormalities in polycythemia vera, having a high hemoglobin or hematocrit alone does not mean a person has PV. Other, more common reasons exist for high levels, such as sleep apnea, smoking, or certain medications like testosterone. It is important to emphasize that high hematocrit does not equal PV.
Initial Patient Conversations
Stephanie: How do you like to approach your first conversation with a new PV patient?
Dr. Fleischman: I want to understand the patient’s personal philosophy about their health and what they would like to achieve with their PV care moving forward. Physicians should not assume their values are the same as those of the patient. People are at different stages in their lives and may have different goals.
For example, an 18-year-old will have different health goals than an 80-year-old. I always ask, “What would you like to accomplish with your treatment?” PV can be managed in many ways. There is no absolute right or wrong approach; it should be personalized. Once I understand the patient’s goals, I provide all available information and then they select the best approach for themselves.
I always ask, ‘What would you like to accomplish with your treatment?’ PV can be managed in many ways. There is no absolute right or wrong approach; it should be personalized.
Dr. Angela Fleischman, MD, PhD, Hematologist and MPN Researcher
UC Irvine Chao Family Comprehensive Cancer Center
Discussing Treatment Options and Patient Preferences
Stephanie: How do you discuss research and treatment options and listen for cues about patient priorities?
Dr. Fleischman: There are common themes among PV patients. Some, especially younger patients, are hesitant about medications and prefer a medication-free approach, often relying on phlebotomy and aspirin, if appropriate. Others want to be proactive, addressing the root cause and aiming to reduce their JAK2 percentage; interferon alpha is frequently discussed for this approach.
A third type of patient simply wants an easy regimen without concern for underlying issues; for them, hydroxyurea might be most appropriate. The treatment style should fit the patient’s preferences.
Latest Advances and Disease Modification Strategies
Stephanie: How do you introduce the latest advances to proactive patients, especially regarding disease modification?
Dr. Fleischman: In polycythemia vera, if the patient is interested in data and disease modification — changing their disease trajectory and possibly eliminating JAK2 mutant cells — the conversation centers on interferon alpha (IFNα). Many patients arrive specifically to discuss interferon alpha, having researched it themselves.
Not every symptom is caused by PV. Over-attributing symptoms risks overlooking other health issues.
Dr. Angela Fleischman, MD, PhD, Hematologist and MPN Researcher
UC Irvine Chao Family Comprehensive Cancer Center
Simplifying the Biology: Why Do PV Patients Have Too Many Red Blood Cells?
Stephanie: Can you summarize why PV patients overproduce blood cells?
Dr. Fleischman: Our bodies need to maintain equilibrium in blood cell production. Hormones signal our bone marrow when to produce more red blood cells or platelets, and this signaling runs through JAK2. A JAK2 mutation, seen in almost all PV cases, causes blood stem cells to always receive the signal to produce more blood cells, leading to excess red cells, platelets, and white cells.
Tracking Symptoms Effectively in PV
Stephanie: How do you help patients track symptoms and understand their importance?
Dr. Fleischman: Symptoms are important and associated with PV, but not every symptom is caused by PV; over-attributing symptoms risks overlooking other health issues. For patients undergoing phlebotomy, it is crucial to track whether symptoms correlate with their blood counts or treatments.
For example, do symptoms worsen before phlebotomy and improve after? Did symptoms resolve when platelet counts dropped on hydroxyurea? Coupling objective data (like blood counts) with symptoms helps clarify what is truly related to PV.
Document symptoms in a journal or other format, noting onset, frequency, and patterns… Even more important is communicating the reason for sharing a symptom.
Dr. Angela Fleischman, MD, PhD, Hematologist and MPN Researcher
UC Irvine Chao Family Comprehensive Cancer Center
Communicating with PV Specialists: Tips for Patients and Caregivers
Stephanie: What guidance do you have for patients and caregivers on communicating with their PV doctors?
Dr. Fleischman: It is valuable to document symptoms in a journal or other format, noting onset, frequency, and patterns, such as whether something happens every Friday or daily for a month. Even more important is communicating the reason for sharing a symptom. Are you reporting it because you want it resolved or simply to inform your doctor? This distinction helps me know how I can best support my patients.
Hopes for the Future: The PV Treatment Landscape
Stephanie: What are your hopes for PV patients in the coming years? What is changing?
Dr. Fleischman: The landscape is changing across several aspects: symptom management, blood count control, blood clotting, and tackling the underlying disease. New treatments target different areas, but for the core disease, interferon alpha remains our best option, as it can bring about molecular remission in some patients.
On the horizon, hepcidin agents (medications that increase hepcidin) will help prevent overproduction of red blood cells but may not address the disease’s root cause. It is important to clarify what “treatment” means, as it can refer to very different approaches. Ultimately, PV is a chronic condition and patients should be empowered to take the driver’s seat in managing their care.
Ultimately, PV is a chronic condition and patients should be empowered to take the driver’s seat in managing their care.
Dr. Angela Fleischman, MD, PhD, Hematologist and MPN Researcher
UC Irvine Chao Family Comprehensive Cancer Center

Inspired by Jeremy's story?
Share your story, too!

Thank you to PharmaEssentia for supporting our independent patient education program. The Patient Story retains full editorial control over all content.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make informed treatment decisions.
The views and opinions expressed in this interview do not necessarily reflect those of The Patient Story.
More Polycythemia Vera Stories
No post found