The Latest in Multiple Myeloma
Understanding Promising Treatment Options
Edited by:
Katrina Villareal
Multiple Myeloma:
Symptoms & Signs
PERSEUS Trial
IsKia Trial
Quadruplet Therapy
MAIA Trial
CAR T-Cell Therapy
Bispecific Antibodies
BLENREP (Belantamab Mafodotin)
Impact of Dexamethasone (Dex) Dose
In this discussion, patient advocates Cindy Chmielewski and Jack Aiello speak with multiple myeloma experts, Dr. Rafael Fonseca with Mayo Clinic and Dr. Susan Bal with University of Alabama at Birmingham. They discuss the latest coming out of the 2023 American Society of Hematology meeting and share the latest multiple myeloma clinical trials and treatment options.
Learn about new multiple myeloma treatment options, including triplet and quadruplet combination therapies, CAR T-cell therapy, bispecific antibodies, and trispecifics. Find out about the future of belantamab mafodotin (BLENREP), dose reduction of dexamethasone, and the introduction of sonrotoclax, dubbed as the next-generation venetoclax.

Thank you to GSK for its support of our patient education program! The Patient Story retains full editorial control over all content.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider for treatment decisions.
- Introduction
- Overview of Multiple Myeloma
- ASH 2023 Meeting
- Front-Line Therapy for Transplant-Eligible Patients
- Triplet and Quadruplet Regimens
- Possibility of Quadruplet Treatment as Standard of Care
- CAR T-cell Therapy
- Bispecific Antibodies
- Antibody-Drug Conjugates
- BCL2 Inhibitors for Myeloma
- Dexamethasone Dose Reduction
- Final Takeaways
- Conclusion
Introduction
Stephanie Chuang, The Patient Story Founder
Stephanie Chuang: I’m the founder of The Patient Story and, more importantly, I also got a blood cancer diagnosis of non-Hodgkin lymphoma a few years ago.
We have so many shared experiences in what we’re looking for and that’s what The Patient Story is all about.
We try to help patients and care partners navigate a cancer diagnosis primarily through in-depth conversations with patients, care partners, and top cancer specialists.
I want to give special thanks to GSK for supporting our educational program. Their support helps programs like this more available and free for our audience. We want to note that The Patient Story retains full editorial control of this entire program and this is not meant to be a substitute for medical advice.
Our patient moderators Cindy Chmielewski and Jack Aiello will lead this conversation. Cindy and Jack, can you share a little bit more about your own stories and how you became such passionate advocates in this space?


Cindy Chmielewski, Patient Advocate
Cindy Chmielewski: I’ve been living with multiple myeloma since 2008. What brought me to the doctor was very debilitating back pain. The pain was so bad that it affected my job. I was having a hard time standing up to teach. I wasn’t able to take my class to the playground for recess. I had to have other teachers do that.
I went to an orthopedic doctor who, unfortunately, I think was unfamiliar with the symptoms of multiple myeloma because he diagnosed me with degenerative disc disease. It took an additional two years to get the correct diagnosis.
Since then, I’ve had several treatments and I’m now doing very, very well. I’m excited because I’m hearing all these new therapies that will be available to me if and when I relapse.
Jack Aiello, Patient Advocate
Jack Aiello: I was diagnosed with multiple myeloma at the beginning of 1995.
It started with a backache but MRIs and X-rays didn’t show anything wrong according to a specialist I went to see. I ended up taking a blood test and that showed an elevated protein. The specialist said I likely have multiple myeloma and that was the start of about eight years of significant treatment.
Back then, the average life span for myeloma patients with treatment was only 2 to 3 years. I was fortunate to respond to a third transplant except that I used donor stem cells after a couple of earlier transplants and clinical trials didn’t work for me.
I’m extremely appreciative to be alive 29 years later. I think this discussion is awfully important as all of these webinars are for myeloma. In particular, we focus on what happened at the 2023 American Society of Hematology meeting that will affect patients immediately as well as where the research is going. I’m always in favor of making sure I understand what’s going on so that I can help myself if my disease comes back and I can help others when I’m leading our support group or talking with other patients.


Rafael Fonseca, MD
Cindy: Dr. Rafael Fonseca is a hematologist-oncologist at Mayo Clinic with a special interest in multiple myeloma. He also participates in and leads clinical trials that have led to the approval of various drugs for the treatment of myeloma
Dr. Fonseca, what drew you to multiple myeloma, and what drives you most to continue to work in helping myeloma patients?
Dr. Rafael Fonseca: The way I got into myeloma is a little bit of luck but mainly because I had great mentors. The late Dr. Philip Greipp was a wonderful influence. I wanted to do lymphoma, but he steered me into myeloma without knowing what would happen. Those were the days of melphalan and prednisone, which links exactly to what drives me now.
We are very fortunate to be living through these times when so many things are happening in the treatment of myeloma. One of my colleagues, Dr. Ruben Mesa, told me a few years back that we are either a drug away or a combination away from being able to cure a significant fraction of patients. We’re shuffling the pieces on the table, but I think we’re there so that keeps us going every day.

Susan Bal, MD
Jack: Dr. Susan Bal is a hematologist-oncologist with the University of Alabama at Birmingham with a special interest in myeloma and CAR T-cell therapies. In fact, at the recent American Society of Hematology meeting, I was fortunate to see Dr. Bal as a primary investigator present phase 1 findings for the clinical trial of BMS-986393, which is a GPRC5D CAR T-cell therapy.
Dr. Bal, what drew you to myeloma, and what drives you most to continue your work to help patients?
Dr. Susan Bal: Myeloma was always very exciting to me personally. I started my fellowship the year that we saw the first results from daratumumab come about.
Other than the excitement in the field and the possibility of a cure, one thing that drives me most is the meaningful relationships that you can continue to build because our patients live for so long and do so well.
I’m so delighted to be able to work with a great group here and across the country. The myeloma community is strong and we forge onward in our path to curing this condition.

Patients can come to us from any of these avenues and that’s what makes it interesting… It’s a very heterogeneous disease, certainly in terms of presentation and how people do overall.
Dr. Susan Bal
Overview of Multiple Myeloma
What is Multiple Myeloma?
Cindy: Briefly, what is multiple myeloma and what are some of the first signs and symptoms?
Dr. Bal: Multiple myeloma is a plasma cell disorder so it’s plasma cells that have become cancerous. We have white cells, red cells, and platelets. White cells fight infections, red cells carry oxygen, and platelets stop us from bleeding.
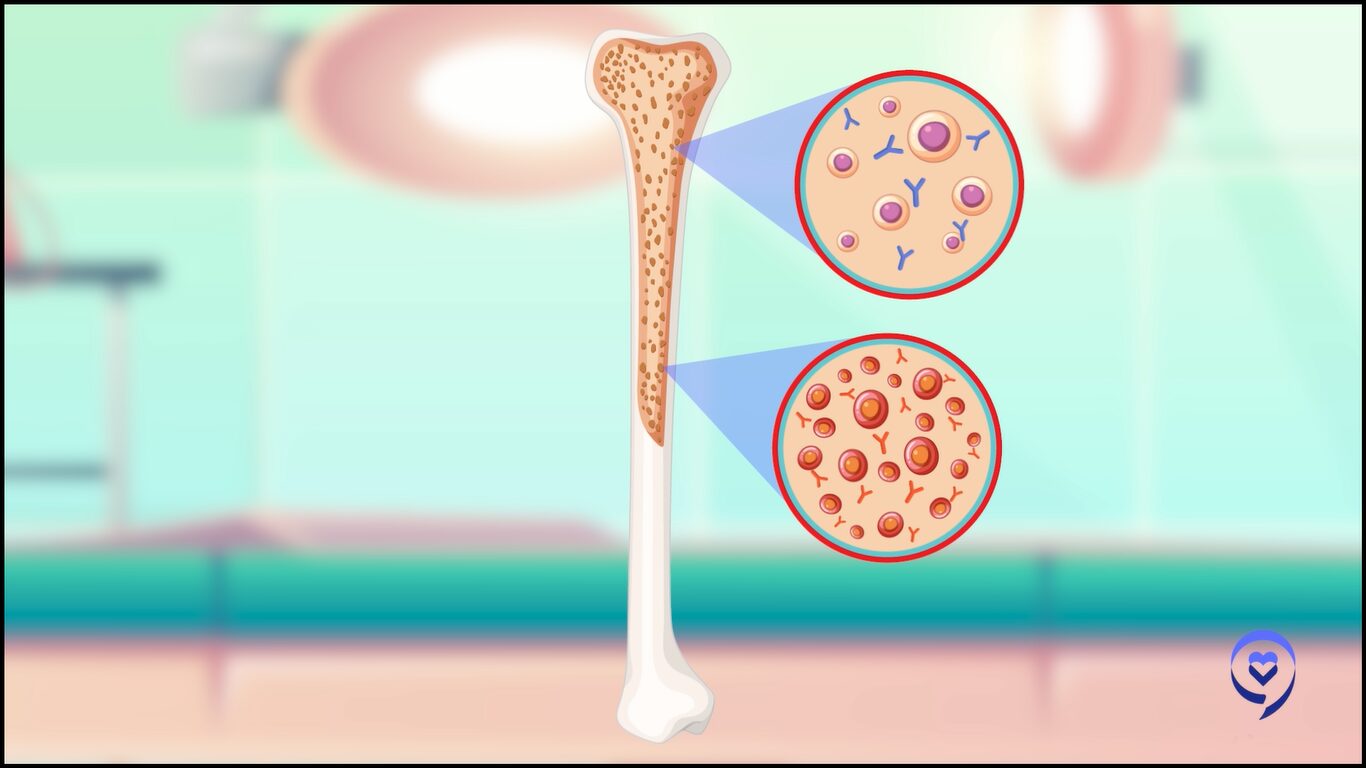
Symptoms of Multiple Myeloma
Dr. Bal: One of the types of white cells that help us fight infection is the plasma cell. The plasma cell is responsible for making antibodies that normally help us fight infection.
Sometimes when these plasma cells go awry and become cancerous, they can crowd out the bone marrow, resulting in symptoms such as elevated calcium levels, bone breakage, and bone damage. It can affect our kidney function, causing renal dysfunction. It can also cause low blood counts because of crowding out of the marrow.
Because it’s a part of the immune system and its job is to prevent infections, oftentimes, patients can present with recurrent infections as a presenting feature as well.

Patients can come to us from any of these avenues and that’s what makes it interesting. We see patients who are simply diagnosed because they had an astute primary care physician who picked up on a protein gap. We have patients presenting on the other end of the spectrum with the plethora of all symptoms that we know as CRAB symptoms, presenting with very disabling features. It’s a very heterogeneous disease, certainly in terms of presentation and how people do overall.
As is true with everything else in medicine, the availability of additional information has allowed us to narrow down the best options for patients.
Dr. Rafael Fonseca
Considerations When Choosing Treatments for Different Patient Groups
Cindy: Dr. Fonseca, we’re lucky now that there are so many treatments available. When I was diagnosed back in 2008, the treatment arsenal was not nearly as filled as it is now.
What are some of the considerations you think about when choosing treatments for different groups, like transplant-eligible versus transplant-ineligible and newly diagnosed and high-risk? What goes through your mind?
Dr. Fonseca: That’s the essence of everything we do in the practice. All of those considerations and how we present them and share them with patients as options for their treatments.
When we started seeing all these drugs come forward and being approved, my first reaction was, “Boy, this is going to get complicated. How are we going to choose? We have all these treatments. My appointments may have to be two hours long because I have to present everything to patients.”
As is true with everything else in medicine, the availability of additional information has allowed us to narrow down the best options for patients. Even though we have many more drugs, we still have 1, 2, or 3 options that are probably the best for a person at a given step. That has allowed us to work more on our messaging and the rationale of what we do.
The considerations you mentioned are critical. We always want to look at, first of all, the patient as a person with their preferences and goals, as well as some other health issues or comorbidities.

One of the critical aspects — and one we can’t modify though I know many wish we could — is our age. Age is a major determinant of how well you’re going to be able to tolerate treatment or not.
Some treatments become quite a bit toxic as we take them later on in life. A great example of this is the stem cell transplant. As physicians and clinical teams, we have to tailor our approach to patients as we start proposing the various treatments.
The second consideration is whether the patient is newly diagnosed or someone who, unfortunately, has myeloma that’s coming back or what we call recurrent myeloma. If we think about that, then we say: which stage is this? Is this an early recurrence or is this someone who has already received 3 or 4 prior lines of therapy?
Then we bring other factors that are important for patients to know about and we discuss them with the patients. We test the myeloma cells for genetic makeup. When we talk about genetics in myeloma, we’re mostly talking about the changes that occur in the myeloma cells, not the genetics that a person may have in the rest of their body and, very importantly, not genetic things that are passed on from generation to generation.
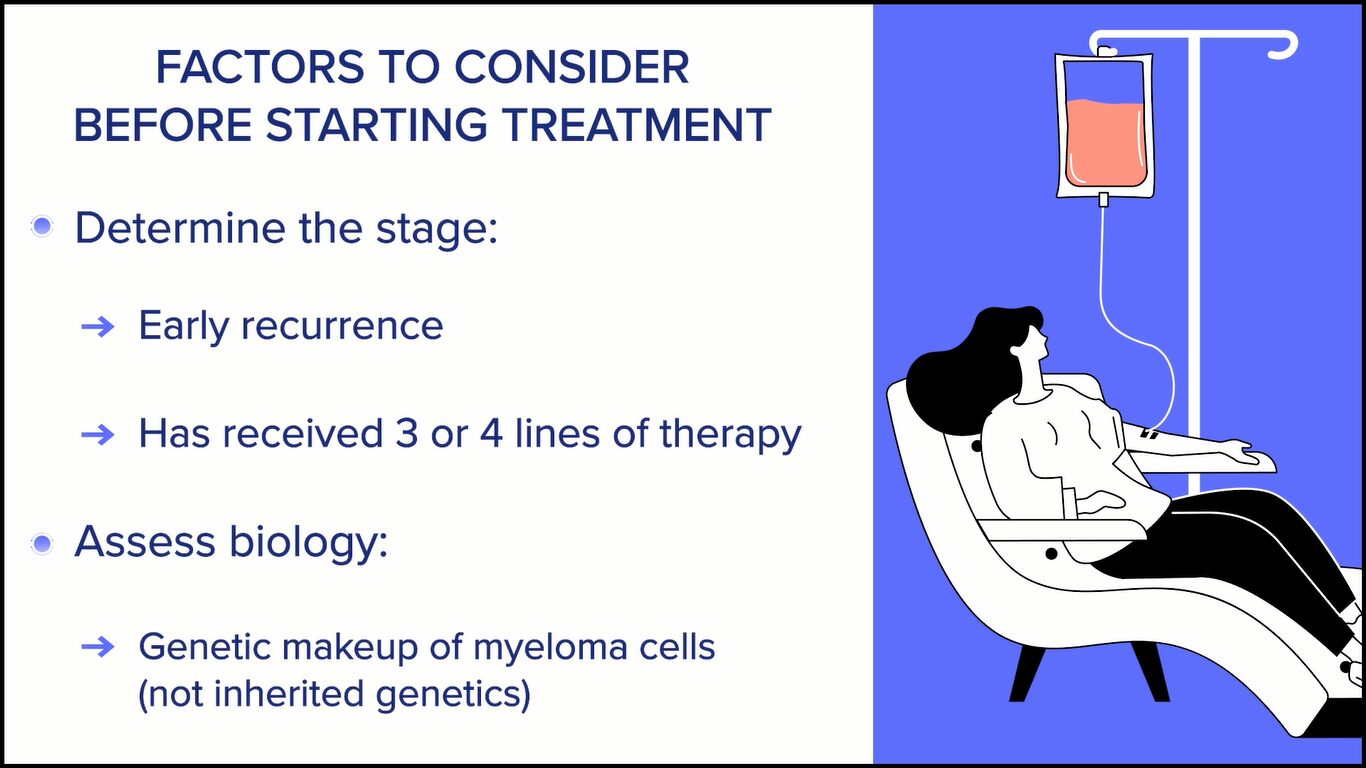
Those are things that play into our decision-making process. Some patients have markers that would make us more concerned, something we call high-risk myeloma. With that in mind, we put together treatment plans that we propose to our patients.
We’re moving forward with a more unified approach to goals. If you go back 15 years or certainly, in 1995, our goal was to control. Can we do something that will put the myeloma at bay that would allow us to gain time?
Many of us feel like the pendulum has swung completely in the other direction. Many individuals, myself included, think that we should aim to eradicate all myeloma cells and, ultimately, produce cures for patients. How you phrase that and plan for that becomes important. Behind that, there’s a whole set of other principles. Those are some of the overriding things we think about but always starting with the patient.
We were lucky to have two major presentations at the meeting, including a plenary session where we saw results from the IsKia trial, as well as a late-breaking session where we saw results from the PERSEUS trial.
Dr. Susan Bal
ASH 2023 Meeting
Biggest Takeaways from ASH 2023
Jack: Dr. Bal, can you explain the importance of the American Society of Hematology meeting and what, from your standpoint, were the biggest takeaways?
Dr. Bal: The American Society of Hematology meeting is a key event for hematologists. It’s where we expect to see some of the latest and greatest of science and all the discoveries and innovations that are occurring within the field. It’s a one-stop shop where we get to see some of the best data so it’s a very looked forward to event in the community.
This ASH was no different. We were lucky to have two major presentations at the meeting, including a plenary session where we saw results from the IsKia trial, as well as a late-breaking session where we saw results from the PERSEUS trial.
The PERSEUS study was perhaps one of the most awaited trials in the sense that this was a randomized phase 3 trial evaluating a quadruplet regimen of daratumumab, bortezomib, lenalidomide, and dexamethasone versus the same combination without daratumumab in patients with newly-diagnosed, transplant-eligible multiple myeloma. For many in the field, we have moved towards using these quadruplets based on an earlier phase 2 randomized GRIFFIN trial. This was practice-affirming and perhaps practice-changing for a small proportion of patients in the community.
What this trial did was take patients who were transplant-eligible — who are fit and in the European and Australian countries where this was predominantly performed — and were randomized into receiving four drugs versus three drugs followed by stem cell transplant and then a doublet maintenance following consolidation therapy with the same regimen.
Overall, what we saw was with a close to four-year follow-up, patients who received this four-drug regimen did a lot better and had longer progression-free survival at the four-year time point. Patients had a very deep response with this regimen achieving measurable residual disease negativity, both using very sensitive markers and sustaining over prolonged periods, which has been shown to be hopefully a surrogate for improved patient outcomes overall.
Within the US, many in the field have already taken up the practice of adopting this four-drug regimen based on the prior GRIFFIN study, but I think this was confirming and affirming for those of us who are doing that and practice-changing for those who were still using the triplet combination. This was perhaps one of the most exciting things to come out of this trial, among other things.
Jack: That’s fantastic. Dr. Fonseca, what about you? Can you describe an outcome you saw from ASH that was so important to the myeloma community?
Depending on how you measure and what the threshold is, we have studies showing that somewhere between 60 and 80% of patients can get into minimal residual disease.
Dr. Rafael Fonseca
Dr. Fonseca: What Dr. Bal described is the crux of what we saw at the 2023 ASH meeting. This is our key meeting where we see all this data.
No one would be surprised if we tell you we actually have a lot of fun being at that meeting because we’re with friends. We work the longest hours that we work in the year. Sometimes it’s hard to convince our family that it’s work because we’re all smiling, talking, and exchanging ideas, and then we go for dinner and discuss further.
It’s a wonderful time for us and more so given the progress we’re seeing in myeloma. The fact that we now have very strong data for what needs to be done in the front line is very important for patients.
At this moment, it’s beyond any doubt that we need to use the best treatments up front. For now, that means the incorporation of anti-CD38 monoclonal antibodies. Dr. Bal mentioned the use of daratumumab. We also have a similar molecule, isatuximab.
The clinical trials point to outstanding results, which could not be seen ever before in the treatment of myeloma. The results are so good that they’re now going to start challenging some of our assumptions.
We had the meta-analysis for the maintenance strategy for myeloma, where patients go through induction, get the transplant, and are placed on maintenance. But Dr. Luciano Costa has pointed out repeatedly that those maintenance studies were done where we could achieve a complete response in about 30 to 40% of patients, minimal MRD negativity, and sometimes patients were getting two drugs as induction.
One would say it was no surprise that more treatment was effective. Whether we call it maintenance or consolidation is semantics. Now we’re getting to the point where many patients have a complete response. Depending on how you measure and what the threshold is, we have studies showing that somewhere between 60 and 80% of patients can get into minimal residual disease.
We’re starting to ask: do you even need maintenance in those patients? I don’t claim in any way that we have the answers, but it reflects how fast things are moving forward. Breaking it down, as Dr. Bal did, is critical.

For some people, we were practicing this, but for other people, either they need this for regulatory purposes across the globe or their reading of the medical literature is such that they want to have that phase 3 trial before they change clinical practice.
Right now, it would be rare that a patient would not be offered induction therapy with something, as we were describing. A lot of things are getting simplified. In a conversation with colleagues, I said the treatment of myeloma should always start with something like daratumumab, lenalidomide, and dexamethasone.
The secondary question is whether the patient needs to get a proteasome inhibitor, like bortezomib or carfilzomib, and whether they need to get a transplant. But then things get simplified because the best players rise to the very top of what we’re doing so I’m excited for what this means for patients.
Dr. Bal: I completely agree with Dr. Fonseca. I feel like some things are almost becoming cleaner as we go. I remember this discussion about three years ago where everybody wanted to know: would you give daratumumab to a transplant-eligible patient? The answer is becoming clearer and clearer that you want to use your best drugs upfront and make that first cut your deepest.
Recently, somebody asked me, “Is there a patient whom you would not give daratumumab to?” I said, “I don’t know who that would be because it’s such a well-tolerated, effective therapy.” Now I’m thinking more like what Dr. Fonseca said about which of the other agents you could potentially get rid of or improve upon, rather than the daratumumab.
I’m not saying we’re ready to dispense with transplants, but the data continues to show that deep responses, no matter how you get there, will result in better outcomes.
Dr. Rafael Fonseca
Front-Line Therapy for Transplant-Eligible Patients
Jack: Dr. Fonseca, one of the important trials — and Dr. Bal referred to it earlier as having been given special recognition at ASH as a plenary session — was the IsKia clinical trial, which compared carfilzomib, lenalidomide, and dexamethasone, adding isatuximab to it or not as front-line therapy for transplant-eligible patients. Isatuximab is like daratumumab in that they’re both CD38 monoclonal antibodies. It was another four-drug trial. Can you talk a little bit about that?
Dr. Fonseca: The reason something is given that plenary status is when the reviewers and the reviewing committee feel that there is something of such high value that reaches the high standards of evidence or that even potentially becomes immediately practice-changing.
This particular clinical trial was one more point in that direction where they started looking at the response rate and the MRD negativity rate for those patients. MRD negativity refers to measurable residual disease or minimal residual disease, which is nothing different than saying we have better markers to assess the depth of a response through mechanisms such as genetic testing.

It’s becoming increasingly clear and very well-documented that achieving the status where you can no longer find measurable disease — using very sensitive tools where some can go at the level of detecting one cell out of a million — becomes a harbinger of great outcomes for patients, of durable responses, and ultimately improvements in the various metrics that we call survival. The IsKia trial shows that.
It’s very interesting to me for a couple of reasons. This is a different antibody, a different molecule than daratumumab, but the presumption by those in the field has been that we should see similar results. These antibodies carry nothing of what we call a payload. They’re a naked antibody so they go and bind to those cells. By doing that, they unleash our immune response to those cells. They’re able to kill more cells. We know that because the responses are better and the MRD is better.
In that particular trial, we have to wait a little bit longer to see over time what the outcomes will be on those metrics that people hear about, like progression-free survival and overall survival. But given what I said about MRD, all the indicators are that this will be a very useful approach for the treatment of patients.

Everyone should get a CD38, like lenalidomide and dexamethasone, at least for the time being. But then the question is: what about these drugs that we call proteasome inhibitors? In this particular trial, they used carfilzomib. For me, that’s very interesting because that’s what I do in my clinical practice.
When we make decisions in our clinical practice, there are trade-offs. Carfilzomib is a sister medication of bortezomib. They’re both proteasome inhibitors, but each one has different toxicities.
There’s no clear-cut evidence that one is better than the other, but I don’t like the enduring toxicity that occurs with some of the other drugs, like bortezomib. That’s where we will be spending our time, talking about those types of drugs. Should we use bortezomib or carfilzomib? Both are standard of care and excellent treatment. There are nuances of why we choose one or the other.
There will be the question of transplant, too. If you have these regimens that can make patients have such a great response that they become “MRD negative,” the question will come forward: do you even need a transplant?
I’m not saying we’re ready to dispense with transplants, but the data continues to show that deep responses, no matter how you get there, will result in better outcomes. Maybe some patients will elect to say, “I’m going to collect cells and think about a transplant later.” That’s a very long, nuanced conversation but a reflection of the progress we’re making with this type of combination.
In terms of which proteasome inhibitor is the right choice, some of that depends on the patient’s profile, comorbidities that they already have, and age.
Dr. Susan Bal
Triplet and Quadruplet Regimens
Cindy: Dr. Bal, you were saying that things are becoming cleaner, but now, we’re thinking about four drugs upfront, but which four drugs? Dr. Fonseca was alluding to it, but are there any considerations that you take into whether your proteasome inhibitor is going to be bortezomib or carfilzomib, or what CD38 monoclonal antibody to use? What goes through your mind now that you’re picking four drugs?
Dr. Bal: As Dr. Fonseca mentioned, the patient in front of you helps determine some of that. As you know, daratumumab and isatuximab are both anti-CD38 monoclonal antibodies. They are both very effective drugs.
However, one is subcutaneous so administration is a little bit easier with daratumumab, which is given as a short injection underneath the skin. The other one requires an IV. For now at least, until we have the subcutaneous version for isatuximab, I do believe that reduces share time and and patient inconvenience very much.
In terms of which proteasome inhibitor is the right choice, some of that depends on the patient’s profile, comorbidities that they already have, and age.
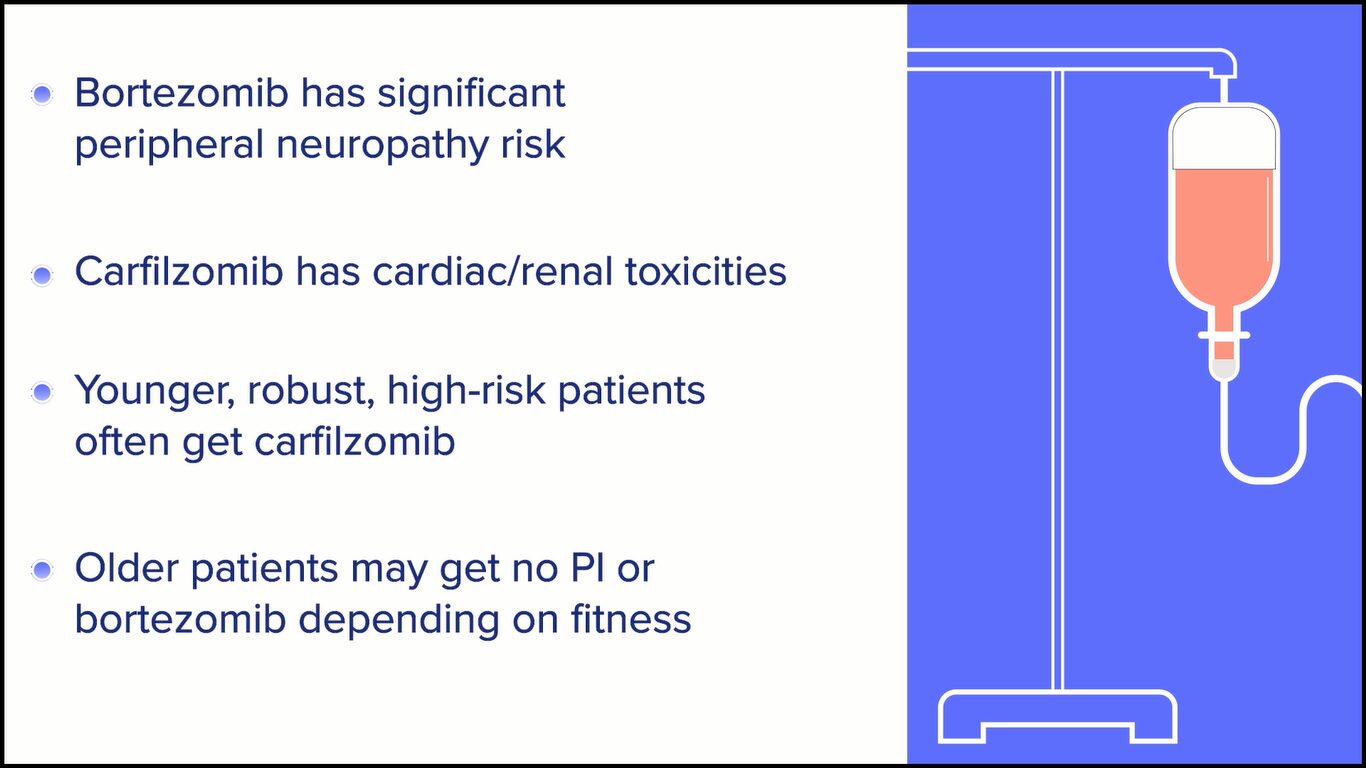
For example, with bortezomib, we’re aware that the risk of peripheral neuropathy is significant and can be quite disabling even at lower grades. However, with carfilzomib, we have seen cardiac and renal toxicities. Even more than just objective data, a lot of people complain of subjective dyspnea and subjective shortness of breath, which can make it challenging for them to feel good during treatment.
Young, very robust, and perhaps high-risk patients are best stratified into the carfilzomib arms, and older patients, depending on their fitness, would then get either no proteasome inhibitor or a bortezomib-based option.
That part is rather individualized, without any clear data of one being overly more efficacious than the other, at least based on the data that we do have in front of us.
In very elderly, very frail patients, daratumumab is exceedingly both efficacious and quite safe, as long as you provide them with appropriate antibiotic coverage, close monitoring, vaccination, and perhaps even IVIG use to protect them from infections.
Dr. Susan Bal
Possibility of Quadruplet Treatment as Standard of Care
Cindy: Are there particular patients that you would not give the quadruplet regimen to, where you would probably go back to triplet therapy or maybe even doublets?
Dr. Bal: Incorporating frailty assessment and assessment of the patient’s overall performance status going beyond the eyeball test that we do is going to be critical in determining who is not fit to receive a quad.
In cases where there are concerns about the use of a proteasome inhibitor, particularly bortezomib, due to underlying diabetes or other comorbidities or the patient’s age and you’re concerned about cardiopulmonary toxicity with carfilzomib, as Dr. Fonseca also mentioned, daratumumab with lenalidomide and dexamethasone is an excellent option with high-level evidence of benefit.

I would say for very old patients who are in their high 80s, even 90s, I would consider a doublet. What’s interesting is that the doublet I would consider would be a dara-dex type of combination or even single-agent dara because IMiDs require hematopoietic monitoring or blood work closely checked for low blood counts. The proteasome inhibitors carry all the concerns that we mentioned.
In very elderly, very frail patients, daratumumab is exceedingly both efficacious and quite safe, as long as you provide them with appropriate antibiotic coverage, close monitoring, vaccination, and perhaps even IVIG use to protect them from infections.
Does everyone need maintenance? I don’t know what the answer to that is yet, but I know that the question is very relevant.
Dr. Rafael Fonseca
Cindy: Dr. Fonseca, do you have anything to add to what Dr. Bal said? What about maintenance? Are you seeing single-drug or double-drug maintenance?
Dr. Fonseca: I want to emphasize one of the points that Dr. Bal made. If you asked me two years ago what I would do with a frail patient who has newly diagnosed myeloma, my response would have been lenalidomide and dexamethasone. However, we saw with the subset analysis in the context of the MAIA clinical trial that the addition of daratumumab significantly improved outcomes for these patients.

I’m glad we have the data because we would be at significant risk of shortchanging patients in the line of thinking of beneficence. Let’s be kind. Let’s use mild treatments. But it turns out that the survival numbers are not as good.
Now, whenever we add a drug, we have to think whether we’re adding toxicity, but the reality is these antibodies are incredibly well-tolerated. It’s different to propose adding carfilzomib or not versus adding daratumumab. Today, I would say the vast majority of patients, even if they’re frail, should get something like daratumumab upfront.
Maintenance is really, really interesting. I’m hoping and I think we’re going to get there soon. Studies, like the ones done by the group of Dr. Bal, are using these adaptive strategies to look at whether we can stop therapy, which naturally leads to the next question. Does everyone need maintenance? I don’t know what the answer to that is yet, but I know that the question is very relevant.
We’re going to get to the point where we’re going to think of maintenance like people think of adjuvant therapy for breast cancer. You complete the surgery. They look at the lymph nodes and the genetic makeup of breast cancer and say whether you need chemotherapy or not. As we incorporate our genetic knowledge, the status of response after induction plus or minus transplant, then we are going to ask those questions.
Does everyone need maintenance? I think it’s going to diverge a little bit more to the extremes. There’s going to be greater contrast. For patients who have standard risk genetic factors, are able to complete induction therapy, have a transplant, and have MRD negativity 10-6, it’s going to be hard to prove that maintenance would have an added benefit. If so, hopefully something that’s better tolerated because, as we know, lenalidomide has significant burden toxicities for patients.
On the other hand, if the patient has residual disease, I don’t think there’s a good enough reason to say that’s what we do. That’s standard. I’m going to cross my fingers and wish that this is not going to come back. I think those are patients for whom we’re going to provide additional treatment for no other reason than to try to get rid of all residual myeloma cells.
I think that’s where we’re going to go with maintenance. I don’t foresee that maintenance, as we have it right now, where everyone gets lenalidomide. You get it until you can tolerate it and hopefully not have side effects or no disease progression. I think that has to change.
If you can eradicate evidence of disease, you can improve your outcomes significantly.
Dr. Susan Bal
Dr. Bal: I couldn’t agree more. We certainly share our opinions regarding the use of measurable residual disease. It’s become the single most important prognostic marker, one that is not defined at all when you’re first diagnosed but comes into play after therapy.
If you can eradicate evidence of disease, you can improve your outcomes significantly. Whether you’re starting as a high-risk patient or a standard-risk patient, getting to that milestone and, more importantly, maintaining that milestone can improve outcomes.
I think maintenance as we know it today will soon cease to exist, hopefully, but mostly because we’re either giving them more therapy, getting them where they need to be, or they’ve done so well because of the excellent therapies we have that we don’t need to continue suboptimal treatments that have other toxicities and risks associated with them.
In the lab, we essentially teach these cells using a viral vector to express receptors on their surface that can target cancer antigens within a patient’s body.
Dr. Susan Bal
CAR T-cell Therapy
Jack: As effective as the treatments are, myeloma still often comes back and one of the exciting new treatments going forward is CAR T-cell therapy. Dr. Bal, can you explain what CAR T-cell therapy is in conjunction with what you presented at ASH?
Dr. Bal: CAR T-cell therapy stands for chimeric antigen receptor T-cell therapy. Within our bodies, there are immune cells and one of those subtypes is T cells, which are normally working to keep our cancers in check. The thought is that these T cells are not working as well as they need to be and the cancer itself is finding mechanisms to evade our immune system, which is what results in disease relapse.
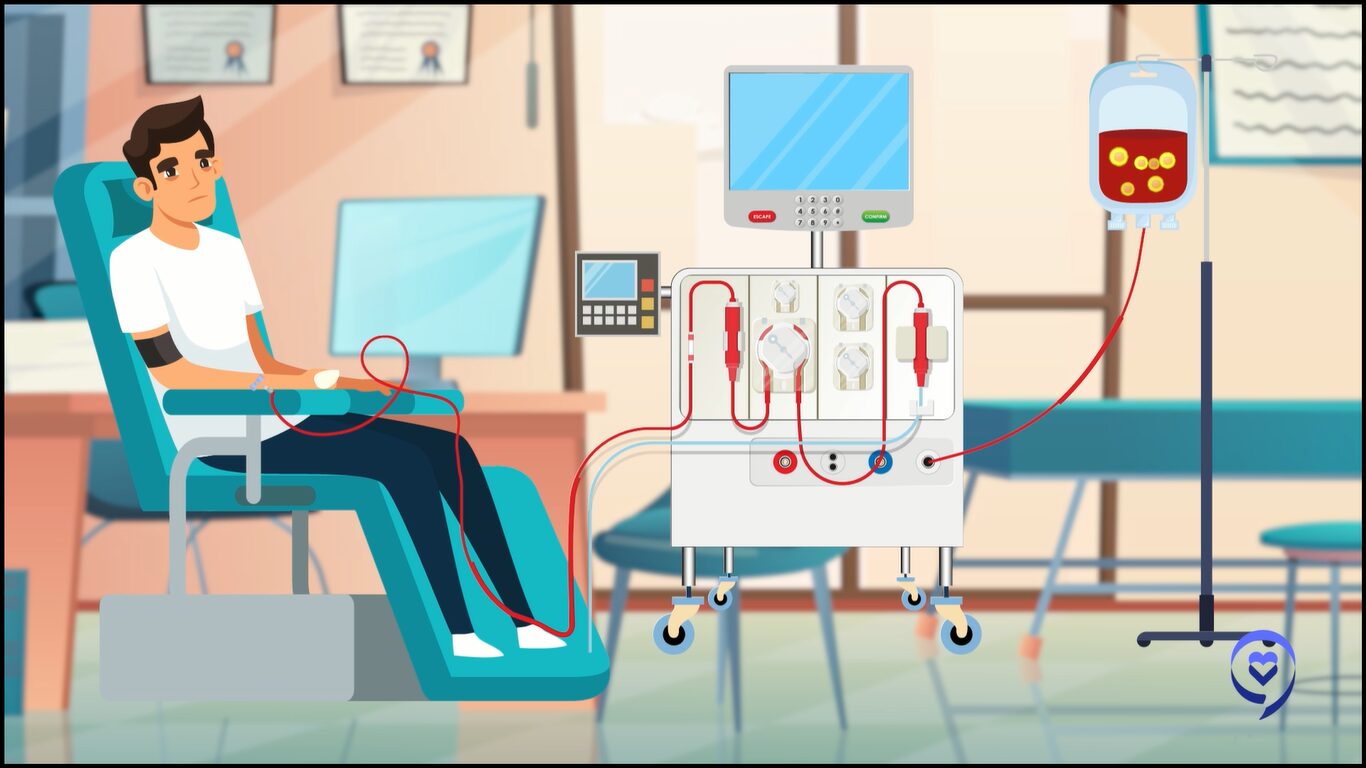
CAR T-cell therapy is a revolutionary therapy. We take out a patient’s T cells by apheresis, very similar to a procedure that patients go through when they do stem cell collection. In the lab, we essentially teach these cells using a viral vector to express receptors on their surface that can target cancer antigens within a patient’s body.
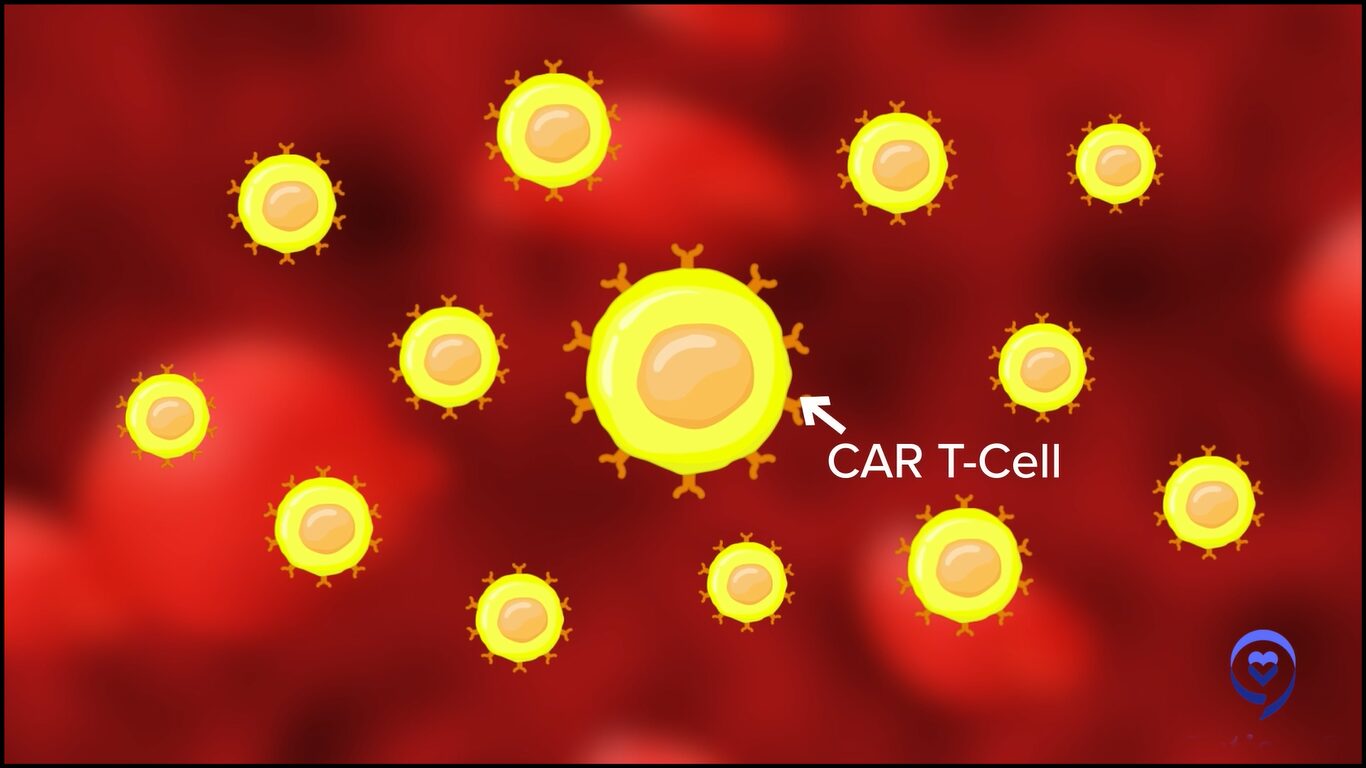
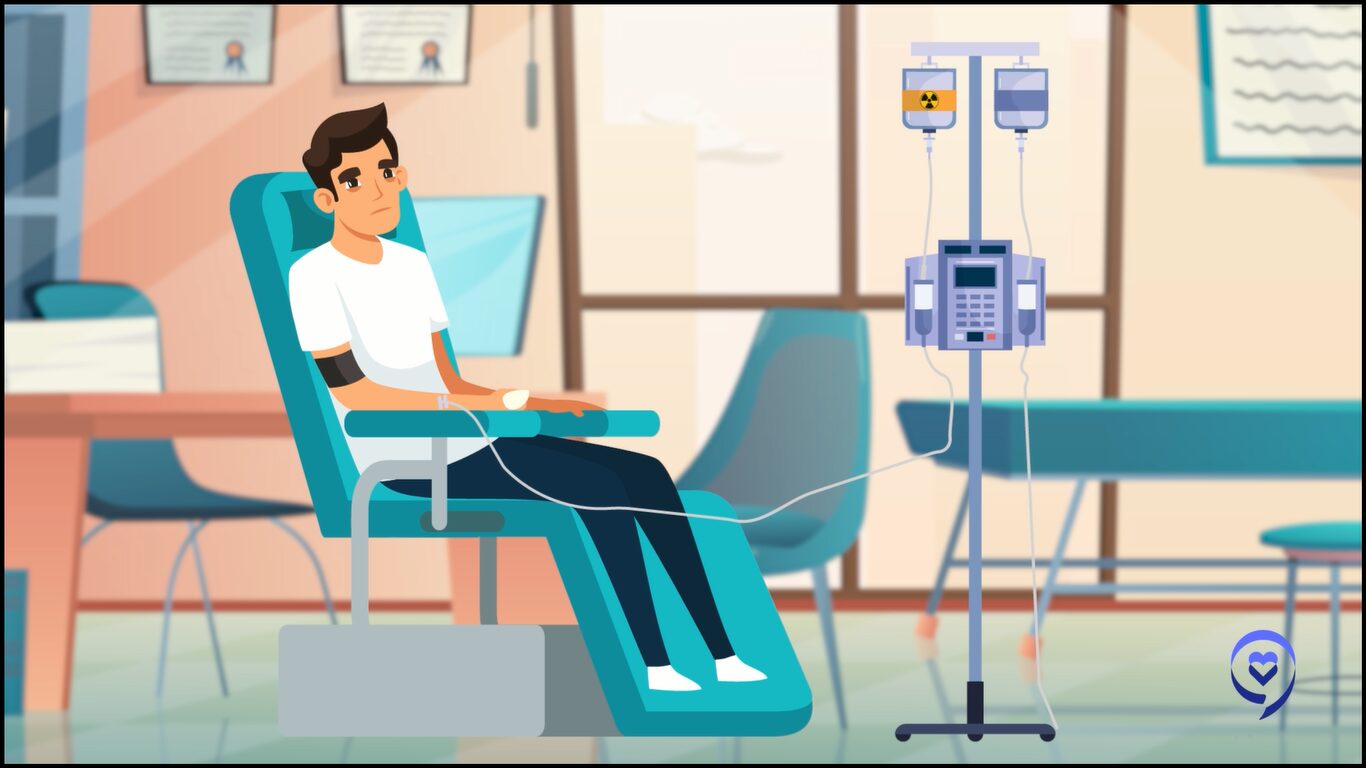
Thereafter, we provide the patients with lymphodepletion chemotherapy, which is essentially chemotherapy that’s given to reduce the number of T cells which are within the patient’s body. We then infuse these chimeric antigen receptor T cells, which go and interact with the antigen, using their CAR receptor, and cause cancer killing or tumor death.
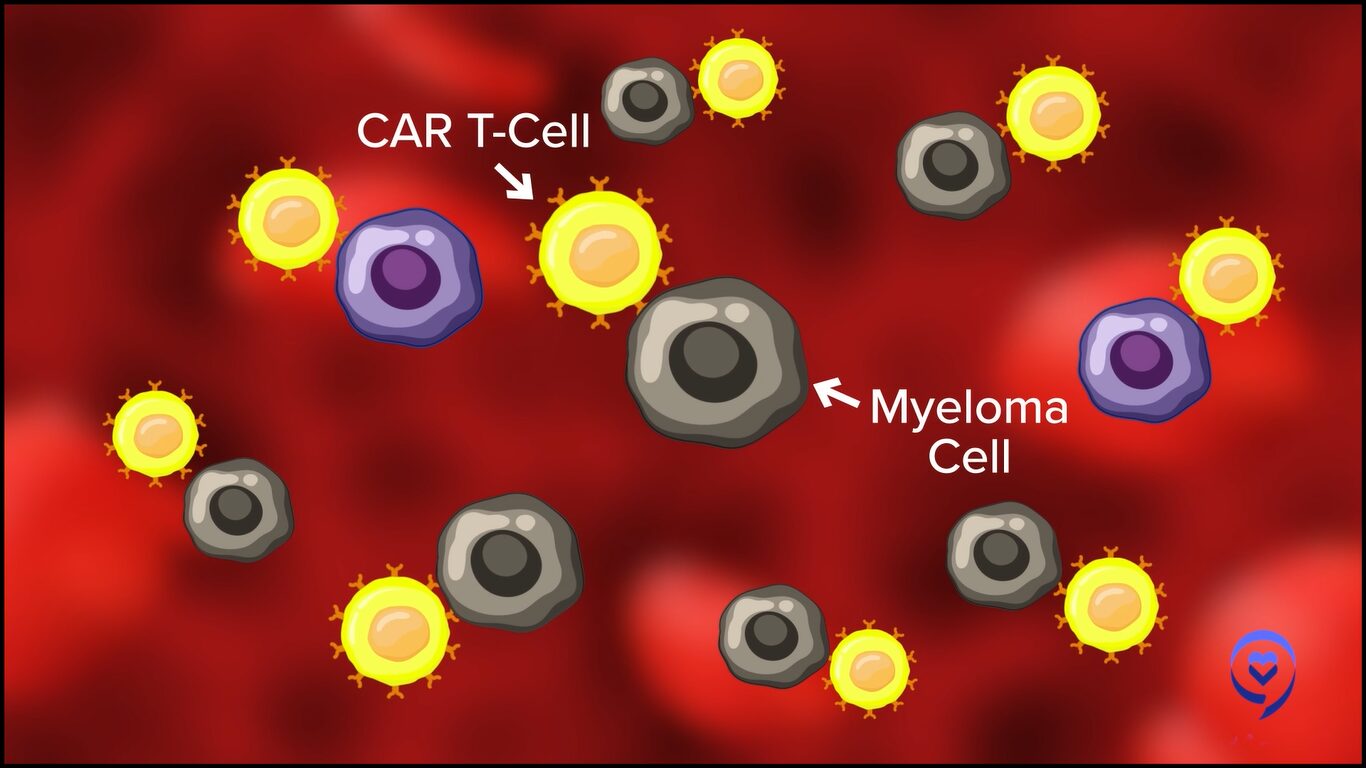
This has been shown to be a very effective modality, particularly in patients who previously haven’t had very good treatment options after they’ve been through the three main pillars of therapy, like CD38 monoclonal antibodies, immunomodulatory agents, and proteasome inhibitors. These patients previously had dismal outcomes measured in single-digit months. Now with these therapies, they’re living longer and having excellent responses, including several that are MRD negative even in late-line disease settings.
At this time, there is FDA approval for two chimeric antigen receptor therapies in myeloma that target the B-cell maturation antigen. This is an antigen that is present on the surface of plasma cells in general and more on the surface of malignant plasma cells or cancer cells. Using this target, we have seen very impressive responses of up to 98% overall responses with one of the FDA-approved agents, known as cilta-cel. This is shown to be an effective avenue.

What we presented at the 2023 ASH meeting is a chimeric antigen receptor T-cell therapy that targets a different antigen known as GPRC5D. This antigen is also one that is expressed preferentially on the surface of these cancer cells and has limited expression across normal tissues, although we do see some expression on tissues such as heart keratinized tissues, on the skin, on nail beds, and in hair follicles. We see some typical side effects, including dyskinesia, with treatments that target this therapy.
Overall, GPRC5D has been shown to be a promising therapeutic target in myeloma. We already have a different immune strategy that’s working against this and is FDA-approved.
In this phase 1 study, we evaluated a CAR T cell that is targeting GPRC5D in patients with relapsed myeloma. What we saw is that in a heavily pre-treated patient population who have received a median of five prior lines of therapy, this was associated with deep responses overall. In about 73 patients who were efficacy available, meaning were available for response assessment, we saw that 88% of the patients had a response.

For the first time, we shared the recommended phase 2 dose, which is the dose that’s going to be moving forward. It was determined at about 150 million CAR T cells. What was encouraging was that the response rate for this recommended phase 2 dose was 91%. At that dose level, we saw low-grade CRS. No patient had grade 3 or higher CRS. There were no cases of other serious toxicities, such as macrophage activation syndrome, etc. This dose was efficacious.
So far, the follow-up for this dose cohort is relatively short so we don’t know what the long-term progression-free survival and overall survival are going to be. But the responses are deep. We need to follow the study longer to understand the more important and relevant outcomes, like progression-free survival and overall survival.
If they work well downstream, as is true for every single myeloma drug, we keep bringing them towards the forefront. The results are quite remarkable.
Dr. Rafael Fonseca
Jack: Dr. Bal mentioned that there are a couple of approved CAR Ts available for patients now, except for the fact that they require four-plus lines of prior therapy.
There are trials and announcements of results from those trials, like CARTITUDE-4 and KarMMa-3, that are testing these same CAR T-cell therapies in earlier lines of treatment. Can you talk about the results in relation to a patient’s quality of life?

Dr. Fonseca: I think that is probably the key aspect of all of this. As you mentioned, two trials are looking at CAR T-cell therapy earlier on: KarMMa-3 with 2 to 4 prior lines of therapy and CARTITUDE-4 with 1 to 3.
What they explored is if they work well downstream, as is true for every single myeloma drug, we keep bringing them towards the forefront. The results are quite remarkable. Many of you saw the presentations and ultimately the publication of the CARTITUDE-4, which was presented at ASCO as well as EHA and published in the New England Journal.
What we’re seeing is this treatment can provide very durable responses that can go on for years for some patients. Then, of course, the natural question is: how does this compare to other treatments? We have effective treatments that can work very well in someone who is experiencing a first relapse, such as the combination of daratumumab, carfilzomib, and dexamethasone or isatuximab, carfilzomib, and dexamethasone.
But the one big advantage of CAR T-cell therapy is that it’s “one and done.” We’re seeing patients who are reporting very high levels of quality of life and making statements like, “I haven’t felt this well in such a long time.” It’s not because CAR T-cell therapy gives you superpowers. It gets rid of all of the side effects that come with treatment.
Even the most benign of treatments involves logistics, medications, blood draws or venipuncture, and some have side effects that come from the chemicals or the biologics that are used. Even in the best-case scenario, it’s something that the patient has to do. The idea that you’re done and all that needs to be done is occasional labs to monitor becomes very appealing.
I think that’s probably going to be the number one driver. Of course, we’re very excited. We’re hoping to see these treatments have very, very long-lasting benefits for some patients. But even if they were not, for some, the ability to be treatment-free would be beautiful.
I always quote our colleague, Dr. Amrita Krishnan from City of Hope. She said that one of the unmet needs in myeloma is to stop therapy so even if it’s temporary, for some hopefully more durable, this would be a way to achieve that.
Jack: I have patients in my support group who say exactly what you say. Some are without treatment three years after their CAR T-cell therapy but even those who relapsed sooner, like within six or ten months, say they would do it all over again because they had that drug-free period that they were so appreciative of.
We know that patients, after having the CAR Ts, can sometimes have prolonged periods of low blood counts and no one really knows why.
Dr. Rafael Fonseca
Side Effects of CAR T-cell Therapy
Jack: That said, Dr. Fonseca, I want to ask another question about a specific CAR T-cell therapy side effect having to do with secondary myeloid cancers, like acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). I’m hearing some concerns about that and wonder what your take is on what’s out there.
Dr. Fonseca: First of all, I’m going to say that we don’t completely understand this and many other factors may come into play.
The FDA recently put an announcement that a fraction of patients, up to 10% in the series that was reported, could develop secondary myeloid malignancies. These are the cells that produce your regular white cells and the conditions that follow from that are either leukemia, acute leukemia, or the preceding stage, which we call myelodysplasia, which is concerning as well to us as physicians.
There are many reasons why this could be nothing more than the reflection of patients living longer and patients who have had exposure before to a number of agents and drugs that are clearly known to cause this. This could include things such as melphalan, which is used for stem cell transplant, but also some of the drugs that are used for the priming of the body for CAR T cells, including fludarabine.
On the other hand, there might be other reasons why this could be linked to CAR T-cell therapy. You’re going to hear more about this as people do the research. For instance, we know that patients, after having the CAR Ts, can sometimes have prolonged periods of low blood counts and no one really knows why.
For instance, in the case of BCMA. BCMA is predominantly expressed in B cells, not in these myeloid cells. How come we have myelosuppression? We don’t know for sure. But if you have that, then there are a lot of hypotheses that can come forward. Does that create an environment that maybe selects for clones that are going to do this? Does that create an inflammatory situation that maybe allows for the progression of some of those pre-malignant clones?
The threat is much greater from unattended myeloma. While we want to have perfect treatments with no side effects, for someone who’s considering CAR T-cell therapy, it’s much better to get it than not, despite these considerations.
Dr. Rafael Fonseca
Our audience is familiar with the term MGUS (monoclonal gammopathy of undetermined significance). As you know, we have myeloma and you have MGUS, and this is a prototype of this stepwise progression. It turns out there’s a similar thing that happens for myeloid cells that there’s something called CHIP (clonal hematopoiesis of indeterminate potential) and it turns out to be very common. It’s one of the things that happens to all of us in life as we age. You develop these premalignant states and CHIP is very common. It can be seen in up to 40% of people.
The question is: what are the CAR Ts doing that maybe makes some of this CHIP grow more or the CHIP cells be favored? We don’t know, but this is an important question.
But practically speaking, as of now, the threat is much greater from unattended myeloma. While we want to have perfect treatments with no side effects, for someone who’s considering CAR T-cell therapy, it’s much better to get it than not, despite these considerations.
Jack: They have other options, like bispecific antibodies.
One of the best parts about bispecific antibodies is the fact that you don’t have this lag time or delay that comes with manufacturing associated with CAR T cells.
Dr. Susan Bal
Bispecific Antibodies
Cindy: Dr. Bal, I hear patients sometimes refer to bispecific antibodies as off-the-shelf CAR Ts and I know they’re not. Can you explain the difference between a bispecific antibody and a CAR T?
Dr. Bal: Bispecific antibodies are antibody fragments. I typically describe them to my patients as a little Y-shaped linker. This is an antibody with two prongs if you will. On one hand, it uses the CD3 molecule to catch the T cell and on the other hand, it has an antigen receptor for one of the myeloma targets.
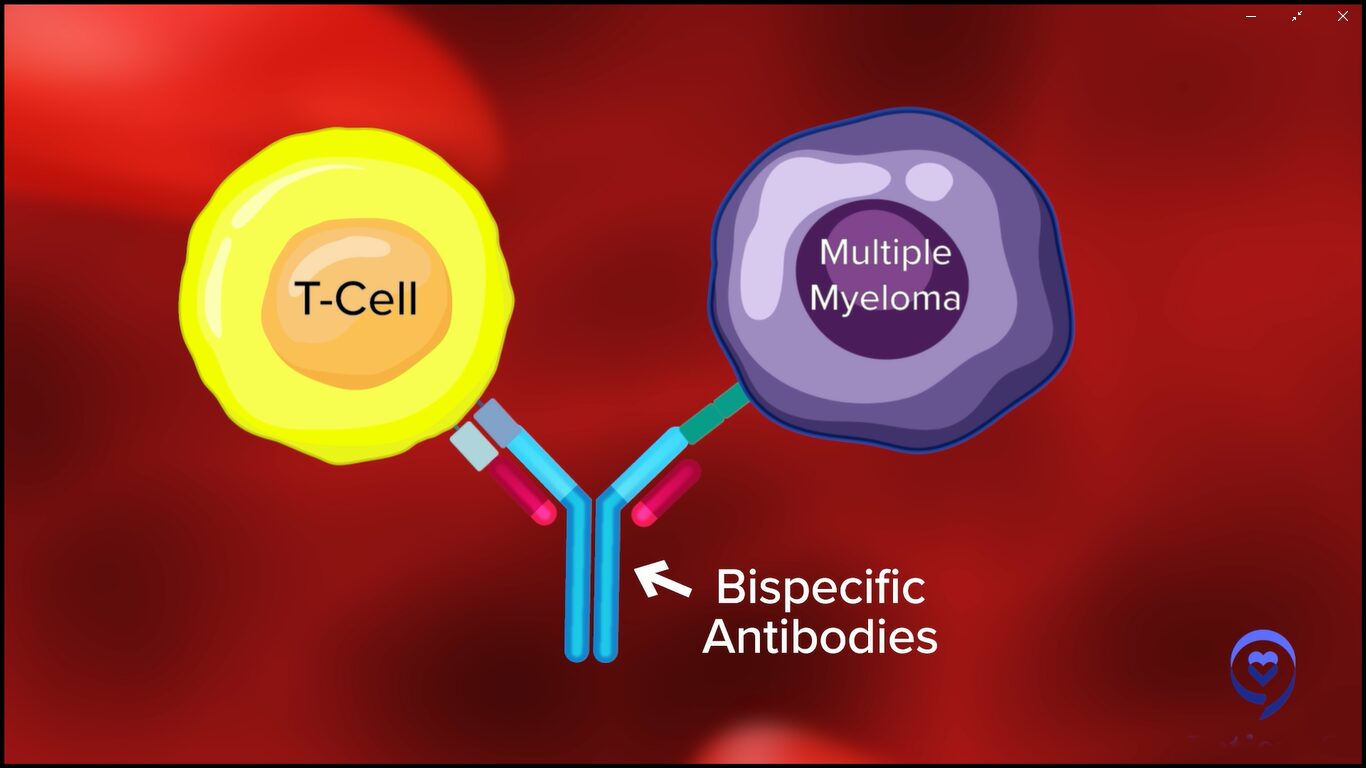
Currently, we have bispecific antibodies that target cancer cells using the same antigens that we talked about for CAR T-cell therapy, such as B-cell maturation antigen (BCMA) or GPRC5D. They target the cancer cell using one of these antigens and bring the two close so that cancer cell killing by the immune system can occur.
One of the best parts about bispecific antibodies is the fact that you don’t have this lag time or delay that comes with manufacturing associated with CAR T cells. These therapies are off the shelf. They utilize the patient’s own T cells, bring them close to the cancer cells, and result in cancer cell killing.

Oftentimes, where we don’t have the time or the manufacturing capability or the slot allocation, or all of those patients who are not necessarily being treated at a large center with CAR T-cell capabilities, this provides an avenue to use new immune treatments and immunotherapies for a wider range of patients. It has the potential to reach many more patients, given the current state of affairs, at least in 2023 and 2024, and so presents a very exciting option for our patients.
Some could argue they don’t have as of yet the same horsepower as some of the CAR Ts. Many of us are thinking of using them down the line, but some of them in combination are incredibly, incredibly active.
Dr. Rafael Fonseca
ASH 2023 Updates on Bispecific Antibodies
Cindy: Dr. Fonseca, were there any major updates on bispecifics at ASH 2023 that we should know about?
Dr. Fonseca: We had a number of updates from some of the longer-term outcomes and some of the studies that are looking at bispecifics in various combinations, including with some of the traditional agents. I’m very interested to see how this plays out.
What we’re seeing is that bispecifics, first of all, are very active. Some could argue they don’t have as of yet the same horsepower as some of the CAR Ts. Many of us are thinking of using them down the line, but some of them in combination are incredibly, incredibly active.
There are clinical trials looking at bispecifics in combination with pomalidomide, bispecifics between themselves, and bispecifics with daratumumab in combination. There’s a trial that combines talquetamab and teclistamab, the RedirecTT-1 trial.
We have new constructs that are being developed. We saw a presentation about something called a trispecific so instead of having one side that a bispecific can attach to, there are bispecifics that could attach to two different anchor points that these myeloma cells have.
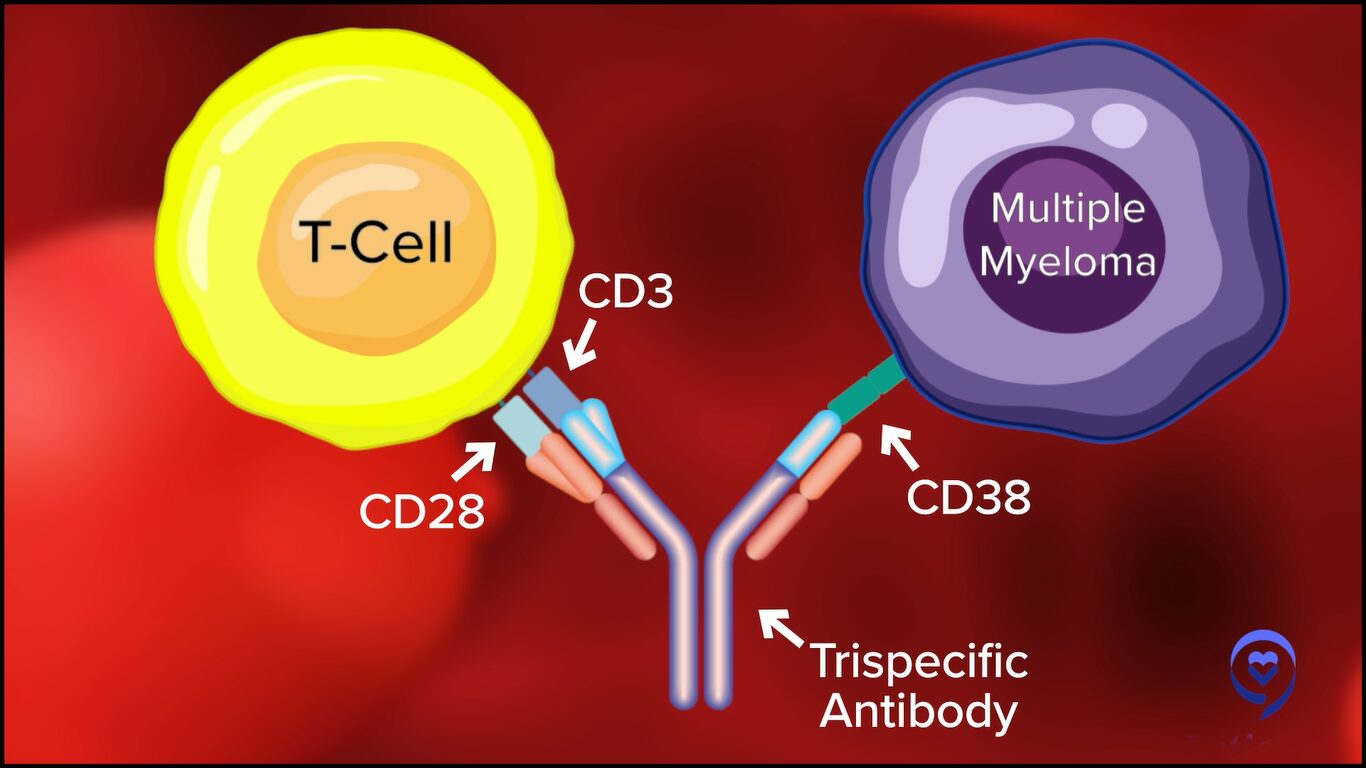
Now, I’m particularly interested in bispecifics because the possibility that many more patients be treated is there for bispecifics than for CAR T-cell therapy. CAR T-cell therapy requires centers of excellence and referral patterns, and there are wait times for cell production. Whereas with the bispecifics, they’re “off the shelf.”
It’s going to take some time. People have to become familiarized with this, but in the future, we’ll see community hospitals and mid-sized hospitals administering these bispecifics, which in my mind is wonderful because many more patients will derive benefit from this type of therapy.
This is the beginning of a new era in cancer treatment because solid tumors are being explored for bispecifics so lung cancer might be treated with this. Oncologists in the future will certainly need to be facile and comfortable with the use of bispecific antibodies.
There’s a lot of push for fixed-duration therapy. One of the greatest reasons, beyond patients having to come to the hospital or clinic and the treatment and travel burden, is the side effect profile.
Dr. Susan Bal
Dosage & Administration of Bispecific Antibodies
Cindy: Dr. Bal, can you talk about the dosing and schedule of bispecifics?
Dr. Bal: Currently, three FDA-approved bispecific antibodies are on the market. We have two agents targeting BCMA: teclistamab and elranatamab. Both target the B-cell maturation antigen. As of right now, these drugs are typically given on an ongoing basis until disease progression or unacceptable toxicity.
Typically, these start in the hospital with a small portion of inpatient hospitalization. Patients receive step-up dosing to minimize the risk of certain side effects. Afterward, they go on to weekly or every two weeks. In this fashion, they go on until the patients continue to respond or come off due to side effects.
However, patients take a median of a little over a month to respond to these agents and up to about six months to get into a complete response. Often, these responses are very deep. In patients who go off treatment because of side effects, we see that some of these patients can maintain their response for years without treatment.

There’s a lot of push for fixed-duration therapy. One of the greatest reasons, beyond patients having to come to the hospital or clinic and the treatment and travel burden, is the side effect profile. These B-cell maturation antigen-directed bispecific antibodies have a lot of infection risk.
Seventy-plus percent of patients get infections and 40 to 50% of these can often be grade 3 or higher, which can be quite serious. These infection risks, particularly oftentimes infection with unusual things that we don’t typically see in myeloma patients, have also enhanced some of our concerns about using these agents on a prolonged basis in patients whose disease may be controlled.
Multiple factors are at play so there’s a lot of interest in studying fixed-duration therapy of these agents to tailor it to a patient’s response and be able to discontinue these therapies once they’re responding to optimize their risk and benefit profiles.
We know that when we use several agents that target the same anchor, there is a possibility that you could develop resistance so we’ll have to wait and see.
Dr. Rafael Fonseca
Antibody-Drug Conjugates
Cindy: Dr. Fonseca, we talked about CAR T-cell therapy and bispecific antibodies. Let’s talk about antibody-drug conjugates. We had one that was approved through accelerated approval and then taken off the market but now it may be back. Could you talk about BLENREP (belantamab mafodotin)?
Dr. Fonseca: Antibody-drug conjugates are antibodies that carry a payload. There’s usually a chemical entity attached to an antibody. In general, what happens is the antibodies bind to the cell surface and go inside the cell. They’re eaten into the cell where they release that chemical structure.
One of those antigens is belantamab. When it first started, it showed very, very high rates of response. Quite promising responses. It went through regulatory approval for a fast-track approval.

Unfortunately, there had to be a confirmatory phase 3 trial that did not meet the endpoint. That means that the data for the trial would not support the complete approval by the FDA. The product has been since withdrawn from the market but is available on a compassionate basis.
This is a highly active compound. It comes with some baggage. One of the main baggage is toxicity to the eyes. Something we call keratopathy and that’s inflammation of the outside layer of the eye, the cornea. As we’re learning how to use it in better doses, that has been a little bit better tolerated. It’s not to say it doesn’t exist, but people are understanding better how to use this.

We learned about two very exciting trials. One of them is the DREAMM-7 trial, which was belantamab, lenalidomide, and dexamethasone versus daratumumab, lenalidomide, and dexamethasone. We’re told the trial is positive meaning there are favorable effects of the belantamab. We’ll have to see what the actual numbers show.
There’s another one that was recently published called the ALGONQUIN clinical trial, which is a combination of belantamab, pomalidomide, and dexamethasone, which has pretty interesting results.
The question is going to be: how do we time all of this? Belantamab targets BCMA as well, too, so that means it could compete with bispecifics and CAR Ts. We know that when we use several agents that target the same anchor, there is a possibility that you could develop resistance so we’ll have to wait and see.
ADCs are one of the hottest things right now in biotech and development for solid tumors as well, too. I heard from people who were at one of those recent conferences that a lot of companies are interested in them. I think we’ll see a comeback of ADCs in myeloma and in other diseases as well, too.
I have patients right now who are still enjoying the benefits of the prior administration of belantamab.
Dr. Rafael Fonseca
Cindy: Do you think Blenrep will be coming back on the market?
Dr. Fonseca: It’s interesting. I’m not currently up to date on what the regulatory pathway will be for that to occur. I think it will, but the results are exciting. The question is how this will compare to some of the other things that I’m mentioning with bispecifics.
I have patients right now who are still enjoying the benefits of the prior administration of belantamab. They have since resolved the eye toxicity. In all cases, with time, the eye toxicity improves. These are patients that may have gotten a short course of treatment with belantamab and remain under excellent control right now.
It’s always better to have more options. I don’t think I would venture out to say exactly how we’re going to sequence this because of the issues of resistance, but I certainly would welcome having that back in our toolkit.
Cindy: I agree. More options are better because we’re also unique and what works for one person may not work for somebody else so I’m glad to have as many bullets in our arsenal as possible.
BCL2 inhibition has a place and hopefully, we’ll find FDA approval soon so we can use it more freely and benefit this unique subset of patients.
Dr. Susan Bal
BCL2 Inhibitors for Myeloma
Jack: Dr. Bal, venetoclax is well-known out there. It’s an approved drug for chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), but it gets used off-label in myeloma patients who have a translocation called t(11;14). At ASH, something called sonrotoclax was introduced as the next-generation venetoclax. Do you have any comments about that particular new drug?
Dr. Bal: Translocation t(11;14) myeloma is a subtype of myeloma that’s a little bit of a different disease itself. It’s a distinct entity and a distinct subtype of myeloma, which responds a little bit differently to different agents. It has a higher dependency on BCL2 inhibition, which is a key part of what drives this myeloma and, therefore, inhibiting it in this space helps drive treatment responses.
We know venetoclax is an effective therapy. Unfortunately, the randomized trials have not worked very much in favor of it. However, those of us who use it and single-arm studies have shown impressive responses in this subset of myeloma patients.
At the ASH 2023 meeting, we looked at a novel BCL2 inhibitor, which is supposed to be at least 10 times more effective and more selective for BCL2 inhibition. It’s called sonrotoclax and they are studying it in a phase 1 setting in several countries. They’re looking to first get the right dose and then expand it in combination with carfilzomib to best understand how these patients respond.
We know venetoclax is an effective therapy. Unfortunately, the randomized trials have not worked very much in favor of it.
Dr. Susan Bal
What we have seen at ASH 2023 was a result from about 19 patients, 10 of whom got the recommended phase 2 dose, which is going to move forward in studies. What we saw was very impressive single-agent activity in combination with dexamethasone at the recommended phase 2 dose of 70%.
What was even more impressive to me was the complete lack of dose-limiting toxicities. There were really minimal side effects that would prevent dose-finding and dose escalation, and a very low risk of low blood counts, which was something that we thought we would see.
The drug was very, very well tolerated. I recall maybe a single patient with high-grade diarrhea and maybe a COVID-19 infection, which was ongoing during the study. Overall, the safety profile is very impressive with responses in combination with dexamethasone of 70% at the recommended phase 2 dose. As the study accrues more patients and studies combinations with carfilzomib, I think it will be exciting.
I will also touch on some of the data that was presented by Dr. Bahlis, which was venetoclax in combination with daratumumab, which showed very impressive responses. Although the patient population was less heavily pre-treated and mostly daratumumab-naive, response rates and measurable residual disease negative states were very, very impressive. BCL2 inhibition has a place and hopefully, we’ll find FDA approval soon so we can use it more freely and benefit this unique subset of patients.
The long-term use of dexamethasone is not contributing much to the control of the disease and we know for sure it’s contributing to the burden of side effects.
Dr. Rafael Fonseca
Dexamethasone Dose Reduction
Jack: Dr. Fonseca, we patients always appreciate it when we learn that doses can be reduced. When we hear that dexamethasone dosing can be reduced, we get giddy about it.
At ASH 2023, there was a presentation that looked retrospectively at a couple of SWOG trials, S0777 and S1211, which looked at patients who had lower dex dosage. Can you talk about that and what that might mean for patients going forward?
Dr. Fonseca: Whenever we discuss treatments with a patient, I usually emphasize that the hardest part is probably going to be the dexamethasone, for reasons you know better than I do. It all started several years ago with a patient, Michael Katz, who said, “We get too much dexamethasone. What can you do about that?” That led to the design of E4A03, which changed our paradigm that we now use dexamethasone at what we call “low doses.”

When my endocrinology friends see those doses, they tell us, “What are you doing? This is a massive dose of dexamethasone.” That was a significant change. We used to use 12 days per cycle, that is 12 out of 28 days at 40 mg of dexamethasone.
We’ve moved forward but have not completely gotten rid of it. From what we know now, as you go on with treatment, as shown by the studies mentioned, as the patient gets stabilized, it becomes increasingly clear that the long-term use of dexamethasone is not contributing much to the control of the disease and we know for sure it’s contributing to the burden of side effects. It should be noted that several studies are showing that. Clinicians need to be cautious and need to adjust the doses of dexamethasone.
In the beginning, most of us feel that patients need a little bit of dexamethasone to “jump start” a good response. But even then, you can do some dose adjustments. In patients of more advanced age, we often will use 20 mg instead of 40 mg. As well, in patients who have amyloidosis, we’ll make adjustments like that.
Is there a way by which we can tell who is going to benefit from the addition of the steroids?
Dr. Rafael Fonseca
It’s important to be mindful of this because patients should not be on 40 mg of dexamethasone once a week for a long time. In general, this is not something that we should be using long-term for maintenance. It can be used short-term for consolidation or other reasons.
The field continues to move in that direction. One patient once asked me, “You always talk about lenalidomide resistance, but you never say dexamethasone resistance. How do you know if it’s contributing down the line?” That is one question that we need to go back and address.
At the beginning of myeloma drug development, there were a lot of people who were interested in resistance to steroids, like Dr. Steve Rosen from City of Hope, and we abandoned that line of thinking. We need to go back and say: is there a way by which we can tell who is going to benefit from the addition of the steroids?
Sometimes, myeloma is very aggressive and very difficult to control. But please don’t assume that because you’re facing this diagnosis, there’s an imminent threat that for sure will be life-limiting because the treatments continue to get better.
Dr. Rafael Fonseca
Final Takeaways
Cindy: If you had one key takeaway that you want patients to hear, what would it be?
Dr. Bal: For me, the most exciting thing in myeloma right now is immunotherapy. The prospect of these new agents that use our immune system to kill cancer cells is really innovative and exciting. I can’t wait to understand how best we can use these strategies to provide fixed-duration treatment and lead to the longest possible remission in a low or undetectable cancer state, such that our patients can have an excellent quality of life.
Dr. Fonseca: We’re very excited and positively so because of the developments in myeloma. We fully realize it would be better to meet you in social circumstances or at coffee somewhere else. But right now, if you’re diagnosed with myeloma, for many, many patients, there are options such that myeloma should not be an immediate threat.
In fact, for many patients, we develop this relationship, as Dr. Bal was saying, and then we track along with you for years and even decades for some patients. It’s not unusual for us to see patients in the clinic well beyond 10 years, sometimes 15 and 20-plus years. I am convinced some of those patients have been cured because of effective frontline therapy. But even for those who haven’t, there are often times options for treatment.
Unfortunately, I know this is not a reality for everyone. Sometimes, myeloma is very aggressive and very difficult to control. But please don’t assume that because you’re facing this diagnosis, there’s an imminent threat that for sure will be life-limiting because the treatments continue to get better.
Jack: For any myeloma patient and care partner, stay educated. There are so many new treatments coming so quickly for myeloma and they are for different target audiences: newly diagnosed as well as relapse patients.
One way to stay educated is to listen to programs like this. Check out the myeloma advocacy websites that offer webinars and videos. Join a support group and make sure to understand the best questions to ask your doctor.
Cindy: It’s important for patients to be seen by a myeloma specialist. Anytime a patient has to have a treatment decision to make, it’s imperative that they get guidance from a myeloma specialist. There are so many new treatments available, new data being presented on different treatments that are now coming to market, and clinical trials so it’s overwhelming.
Conclusion
Stephanie: Thank you so much, Jack and Cindy, for being our incredible patient advocates and moderators. Also to Drs. Fonseca and Bal for the work and research you do to help move the landscape of treatment options in multiple myeloma.
We hope that you walk away with a better understanding of the latest treatments and clinical trials in myeloma. They may or may not be right for you, but it is our goal to make sure you feel empowered to make a decision for yourself.
Thank you and we hope to see you again at a future program at The Patient Story.


Special thanks again to GSK for its support of our independent patient education content. The Patient Story retains full editorial control.