Take Charge of Your MPN
Tracking Symptoms & Advocating for Better Care
Living with an MPN (myeloproliferative neoplasm) brings challenges that aren’t always visible—but tracking your symptoms can make a powerful difference.


Thank you to Incyte and Karyopharm Therapeutics for supporting our patient education program. The Patient Story retains full editorial control over all content.
Edited by: Katrina Villareal
- Introduction
- Meet Our Panel
- What Were the First Symptoms of Your MPN?
- Tracking Your MPN Symptoms
- What Motivates You to Track Your Symptoms?
- Noticing Your Symptom Patterns
- Tracking Symptoms to Better Inform Your Care Team
- How Tracking Symptoms Can Inform MPN Treatment Options
- How Has Tracking Symptoms Changed Your Treatment?
- Dealing with Fears About Your MPN Symptoms Returning
- How Care Partners Can Help You Track Symptoms
- Participating in Clinical Trials
- Considering Clinical Trials in the Future
- Doing Your Own Research on Clinical Trials
- Final Words of Advice
- Conclusion
Introduction
Ruth Fein: This conversation is focused on living with myeloproliferative neoplasms, or MPNs, and how tracking symptoms can empower you to advocate for the care that you need or the care of a loved one.
My story spans over 30 years. I was initially diagnosed with essential thrombocythemia (ET), which transformed into polycythemia vera (PV), and then eventually became myelofibrosis (MF), which is what I have now. I’ve had several symptoms and I’ve been on a few different drug therapies along the way. I’ve had life-threatening clots and life-threatening bleeds, which, of course, conflict with each other.
Now, I’m on a clinical trial and doing great. They call me the poster child for this clinical trial and it’s been five and a half years. I’m always proud and happy to speak to other people living with MPNs, particularly since I didn’t know anyone else living with an MPN for the first 20 years that I had this disease.

This discussion is brought to you by The Patient Story, where our mission is to humanize cancer. We’ve had more than 100 million views of our in-depth story videos that mostly feature patients, sometimes caregivers, care partners, and, of course, physicians, clinicians, and other care professionals. Our goal is to help promote self-advocacy and connection.
We want to thank our sponsors, Incyte and Karyopharm, for their support of our independent educational program. This allows us to create more content like this for free. The Patient Story retains full editorial control. While we hope this is helpful, this is not a substitute for medical advice. Please still consult your healthcare team when making treatment decisions.

Meet Our Panel
Ruth: I’m joined by two other amazing people who also advocate for those living with MPNs.
Nick Napolitano: Hi, Ruth. My name is Nick. I’m a polycythemia vera patient. I was diagnosed in 2016, so I’m approaching the 10-year mark, which is a big deal. Like you, Ruth, I’ve experienced a range of symptoms throughout my journey — everything from itching to bone pain. I have experienced different treatment options and am currently on a drug, doing very well.
I’m a husband to my beautiful wife, Kara. I have two boys, Jake and Nick. They keep me very active.
Demetria J: I’m a wife, a mother, a mother, a business owner, and a coach and mentor. I’ve been a business owner for nearly 20 years. I initially started in the beauty industry and owned a nail salon. Then in 2021, I pivoted to salon suites and an event space. And then life happened.
I received a diagnosis of ET in 2018. I went through a series of different doctors and ended up at Emory in Atlanta, where the oncologist put me on a new medication that had just come out of clinical trials and was showing positive results. He believed that, at that point, I would benefit better from that.
Eventually, my levels began to level out. My platelet counts went down tremendously, so I thought we were in the clear. Then in 2023, I found out it had progressed to myelofibrosis. I was experiencing different symptoms, such as fatigue and stomach pain. I went to the emergency room, was given some pints of blood, and a diagnosis of myelofibrosis after having a bone marrow biopsy.
Ruth: Thank you both for being here. Let’s dive in.


What Were the First Symptoms of Your MPN?
Ruth: We’re talking about understanding symptoms and what to track. One of the main challenges with MPNs is that the symptoms are often subtle and can easily be mistaken for normal aging or other conditions, like menopause. Nick, what symptoms did you notice first? You alluded to a few, but what was the first symptom that prompted you to start tracking?
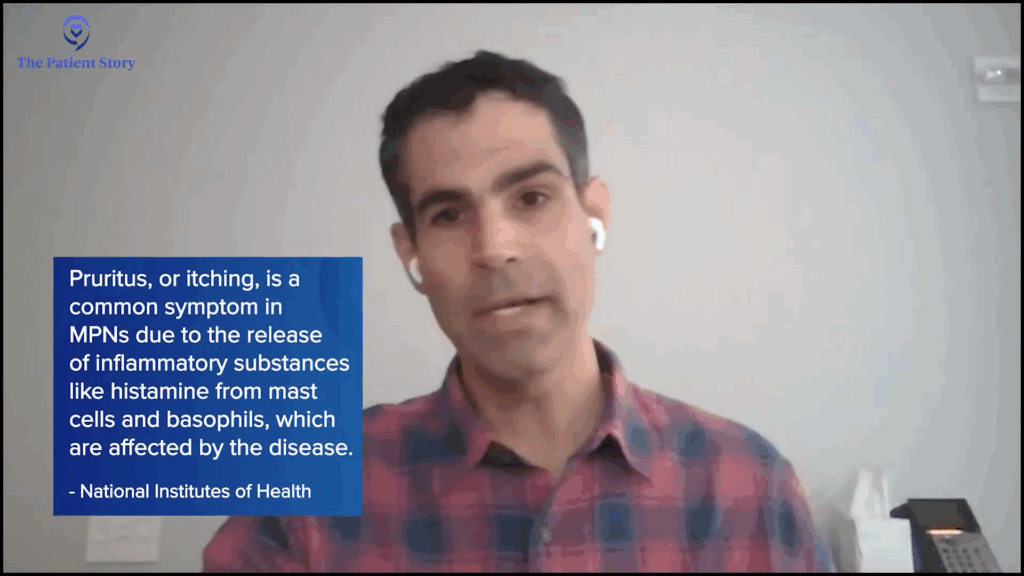
Nick: The itching, for sure. Like you mentioned, a lot of symptoms can be dismissed, like headaches and fatigue, but the itching stood out. It wasn’t something I experienced before, so that raised the red flag and made me start tracking and monitoring the details around when it would happen.

Demetria: Probably the fatigue. I have a high pain tolerance level and the fatigue that I experienced would probably get to most people, but it wasn’t getting to me. My adrenaline was running, being a mom, a wife, and a business owner. I had a very demanding life, so I was going and going, and not paying attention to my body as much as I probably should have been.
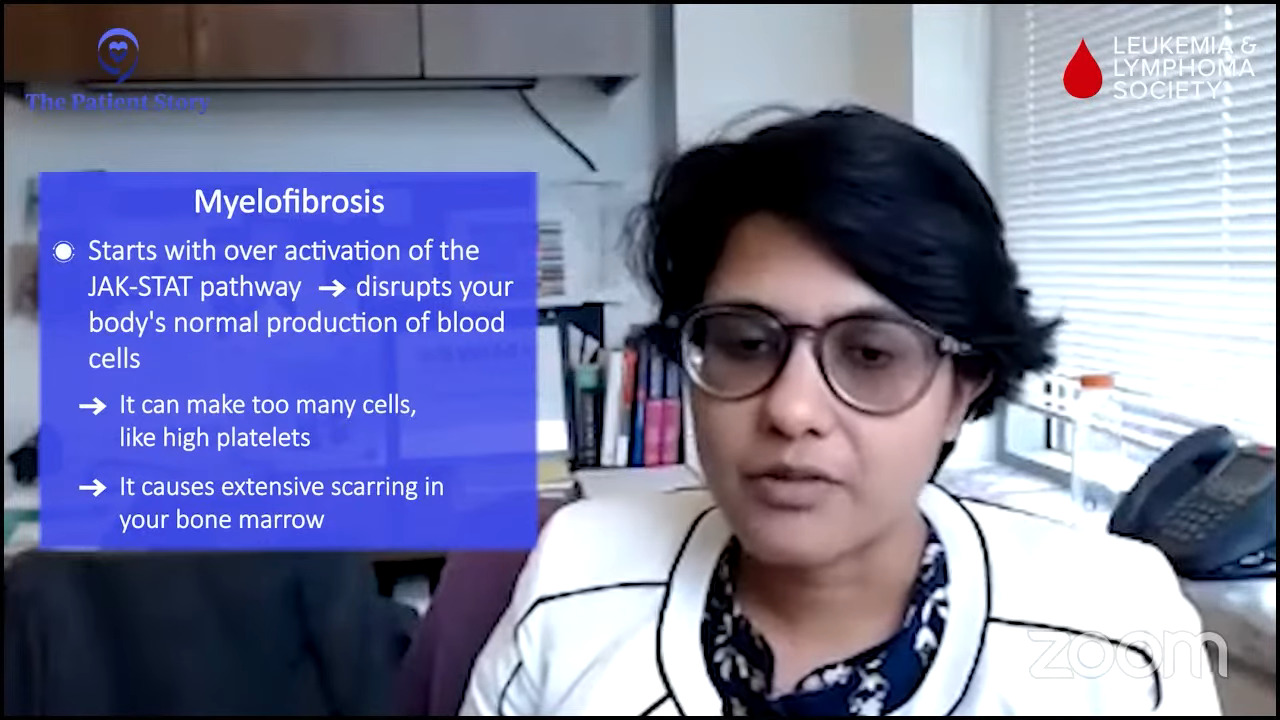
It wasn’t until the day before I went to urgent care. I was having bad stomach pain, which I now know was because of my enlarged spleen. My doctor informed me that when your bone marrow is not making enough white blood cells that the spleen will kick in and try to do the job of the bone marrow and that’s not its job. In the process, your spleen gets enlarged because it’s doing something that it is not designed to do.
That day, I also had some shortness of breath, which was very unusual for me because I usually don’t get out of breath. My body was sending me signs and I ignored them until it gave me a dire warning. “We can’t go anymore. Something needs attention.”
I want to know every little detail about what I’m feeling and then I use that as part of my conversation with my doctor.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
Tracking Your MPN Symptoms
Ruth: What kinds of things do each of you track now to manage your MPN and how has that been helpful?
Nick: I track everything, but that’s me personally. I want to know every little detail about what I’m feeling and then use that in conversation with my doctor. I let my doctor tell me whether I’m crazy or not with respect to the symptoms I’m feeling. But I think it’s important to track everything.
So much of our disease is a journey over time. Things pop up and go away. I think you need to track everything and have the details around it. Make sure you’re communicating with your doctor because you never know what part of the journey or what symptoms you’re experiencing at any given time actually matter until you look at everything.

Demetria: I didn’t track symptoms until towards the end, maybe that last week before I received my myelofibrosis diagnosis. Each day, I was saying, “Wait a minute. I am really, really tired.” I would go to the grocery store, come back home, lie down, and would need to sleep for like an hour and a half.
The other thing I dismissed was a little bit of dizziness because I had just had a sinus infection, so they were thinking it was vertigo from the sinus infection. I thought everything was isolated and not related to one diagnosis. I thought they were all individual symptoms of something else.
The last week before receiving my diagnosis, I was noticing a little bit of progression. The fatigue was feeling a little bit more intense each day until that last day when I had severe stomach pain.

Ruth: How do you track symptoms? Is it through an electronic app or do you use a notebook?
Nick: It’s a combination of an app and a spreadsheet. I would say the most basic and useful tool for me is the notes app on my phone. I open it up almost daily and log stuff there. Then I use that as the basis for my conversations with my doctor. Everything is there between visits, so I can go over questions and things I notice.
It’s the fear of missing something that may play a role in changing the course of my disease or progression.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
What Motivates You to Track Your Symptoms?
Ruth: Nick, what’s helped you be consistent with your tracking? What’s made it easier and what helps you remember to keep it up?
Nick: It’s fear, to be honest with you. It’s the fear of missing something that may play a role in changing the course of my disease or progression. I’m very fearful of not tracking something, going to a doctor’s appointment, and being asked, “Have you experienced this before?” And then having to say, “I don’t know. I haven’t been tracking it.” Then, realizing that it’s too late. Quite literally, it’s fear.
Ruth: That’s real.
Noticing Your Symptom Patterns
Ruth: Let’s talk about symptom patterns. Do you ever notice your symptoms come and go, get worse at certain times, or come in waves?
Demetria: I don’t know that I necessarily noticed any patterns. It was an everyday thing. I was starting to notice that the fatigue was increasing every day. I don’t necessarily know the time of day, whether it was in the morning or the evening. I just knew that when I woke up, I was fatigued. When I went to bed, I was fatigued. Throughout the day, I was fatigued.
It’s unpredictable… You don’t know what’s going to happen from one day to the next when it comes to symptoms.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
Ruth: What about you, Nick? I assume that’s why you started tracking, because our symptoms do come and go. Do you ever notice that they get worse at certain times of the day or on certain days? What have you found in your tracking?
Nick: Yeah, I think that’s probably the most important part of tracking. I’ll give an example with itching. Itching was dormant for a while and then it popped up over the last couple of years. It’s not just random — it happens at certain times of the day. It pops up in the morning and at night, and then it goes away in between. When I take a shower, whether hot or cold, it pops up. When I roughhouse with my kids, the rubbing of my skin activates it. I used to have a lot of bone pain, but that’s gone.
One of the challenging parts of the disease is that sometimes it’s unpredictable. Things come and go. You don’t know what’s going to happen from one day to the next when it comes to symptoms.
Now I feel better prepared because I understand how important it is to pay attention to everything concerning your body.
Demetria J.,
Myelofibrosis Patient & MPN Patient Advocate
Tracking Symptoms to Better Inform Your Care Team
Ruth: I think that uncertainty is palpable. A lot of us experience anxiety and stress related to that. Does tracking ease some of that anxiety because it helps make sense of things, or is it the opposite? Do you see those ups and downs and worry more because of it?
Nick: That’s a great question. It helps me because I feel like I’m educated and prepared when I go to my meetings. I call them meetings with my doctor and I treat it very much like a work meeting. I want to have all the information I possibly can when walking into that meeting. I feel a little more prepared. Having the knowledge of the ups and downs, and maybe the reasons why, is very, very important for me.

Demetria: Yeah, absolutely. One of the things my transplant surgeon pushed for was for me to be very observant of my body, like skin changes, because not only in terms of cancer coming back, but also graft versus host disease with having the transplant. Sometimes your body can reject the transplant. They were huge proponents of me making sure that I am paying attention to everything — not just how I’m feeling, but any changes in my skin, my eyes, my throat, my ears, or my range of motion.
Now I feel better prepared because I understand how important it is to pay attention to everything concerning your body and to make sure that I am letting my care team know, so that we can be ahead of it to be more proactive instead of reactive. Sometimes those subtle things that we don’t pay attention to could absolutely be the start of something. If you catch it early enough, you can stay ahead of it.
Don’t think, ‘Oh, this is probably not related to this.’ Express everything that’s going on in your body.
Demetria J.,
Myelofibrosis Patient & MPN Patient Advocate
How Tracking Symptoms Can Inform MPN Treatment Options
Ruth Fein: Now we’re tracking our symptoms and thinking about when it’s important to share with our care teams. Has that directly led to any action or changes in your treatment? I’d love to hear any examples. We’re not talking about specific drugs, but has it led to a change in your treatment or your activities, Nick?
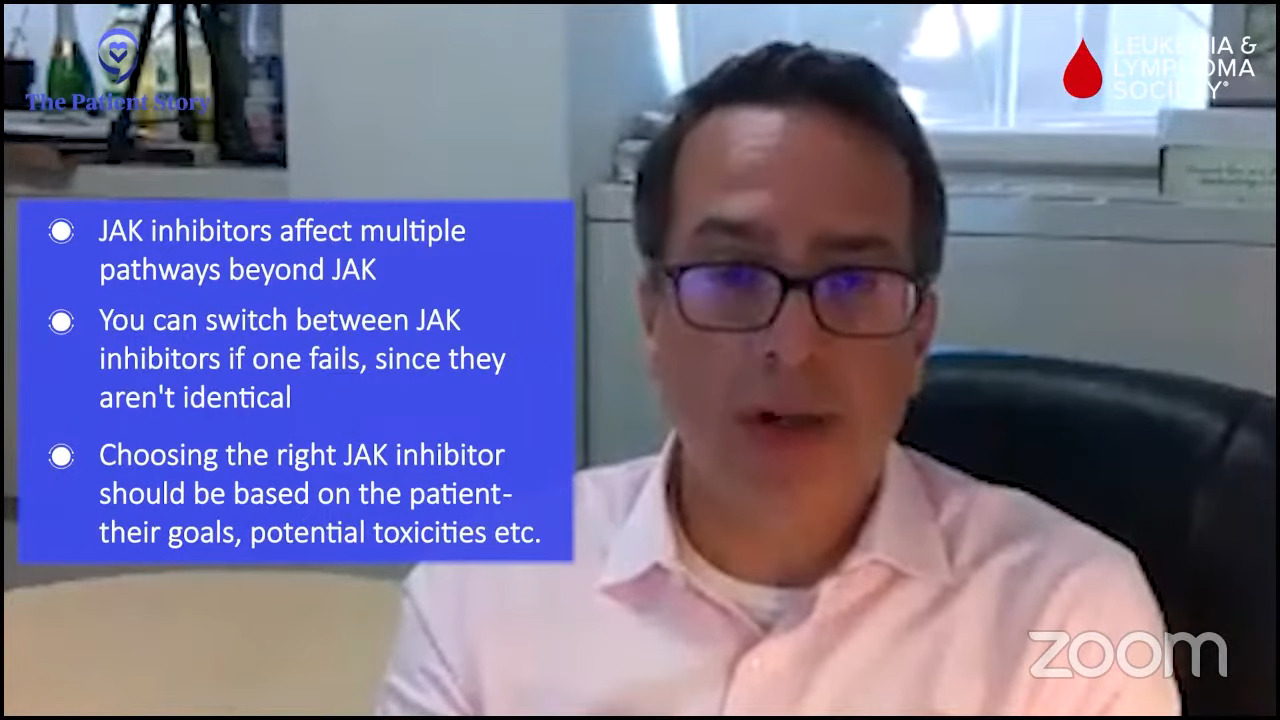
Nick: Yeah, for sure. I talked about the itching and brought that up with my doctor. We talked through the details of when it happens and when it doesn’t. He recommended a couple of different options and one in particular that has worked. I very much appreciated the dialogue and brainstorming. That’s one of the aspects I want from my doctor is brainstorming and idea generation. That’s been a life changer, to be honest with you.

Ruth: Tracking your symptoms and bringing that information to your health team can make a big difference. What tips do you have for talking openly, honestly, and clearly with your doctor and your entire care team about how you’re feeling? Do you have any specific tips? What helps you maintain an open conversation with your care team?
Demetria: One of the things, looking back, is I wish I hadn’t seen my symptoms as isolated incidents. I wish I had known to say to somebody, “I’m having these symptoms,” and list them all out, instead of thinking, “This may be related to the sinus infection. Maybe this is related to this.”
I wasn’t sharing with my oncologist or different people early on about other symptoms that seemed random. I didn’t mention those things when they could have been related to the progression of the disease. When working with your care team, it’s important to tell them everything that you’re feeling. Don’t think, “Oh, this is probably not related to this.” Express everything that’s going on in your body.

Ruth: Nick, do you have anything to add or something that specifically works for you to have an open and honest conversation with your practitioners?
Nick: I make sure that at the beginning of the appointment, we talk about how I’m feeling — mentally, physically, and symptom-wise. I try to talk about how I’m feeling in between visits. I’ve learned that doctors and caregivers want details. They’ll ask about what you’re experiencing and how you’re experiencing it. Coming prepared to the conversation right up front to talk about some of the details of what you’re feeling creates a nice dialogue with your doctor.
Ruth: It sounds like you have a perfect doctor because we’re always talking about how to have productive conversations with your doctor and care team. You’ve got it down and I’m sure our audience will learn something from that.
Nick: Ruth, I learned that over time. Believe me, I learned that over time. It’s not always perfect.
You can learn so much from other people’s experiences and gain real courage and support that way.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
Ruth: Same here. And it’s not easy for everyone. You’re communicative, I’m communicative, but some people aren’t. We always encourage bringing a care partner, someone who can interact on their behalf or listen closely, especially if they might miss something the doctor says. For those who aren’t as outgoing or find it hard to share how they’re feeling, that care partner can step in and say, “Remember last week, when…” or add important details. All of that helps. Thanks for sharing that!
Nick: I totally agree. That’s exactly why programs like this are so important. Connecting with the patient community makes such a difference. You can learn so much from other people’s experiences and gain real courage and support that way.

How Has Tracking Symptoms Changed Your Treatment?
Ruth: As one more follow-up to that, has any of your tracking changed not just your treatment, but raised new concerns that you then brought to your doctor? Questions and answers that perhaps would not have come up before?
Nick: It was the tracking of phlebotomies when I was being treated solely with phlebotomies and the impact that was having on me physically. For a while, I was able to deal with them well, but over time, they affected my overall health and well-being. I wasn’t tolerating them as well as I had in the past, so we started talking about different options. Not only were they not making me feel well, but I was also getting them too often, which is usually a red flag with MPNs. That changed the conversation toward exploring different treatment options for my care.
Ruth: That speaks to how important it is, as a patient or patient advocate. Sometimes doctors ask us yes-or-no questions, like, “Are you doing well with your phlebotomies?” and it’s either yes or no. But if you expand on that and say, “Yes, I’m going, and yes, they’re helping my blood counts, but I’ve been experiencing this or that.” If a person living with MPN or their care partner doesn’t step in to add to that yes-or-no question, we don’t progress in our knowledge and, therefore, better care. Thanks for going there. It’s important for others living with MPNs to hear different experiences.
I’m a woman of faith, so I rely heavily on my belief in God. It has anchored me throughout my journey.
Demetria J.,
Myelofibrosis Patient & MPN Patient Advocate
Dealing with Fears About Your MPN Symptoms Returning
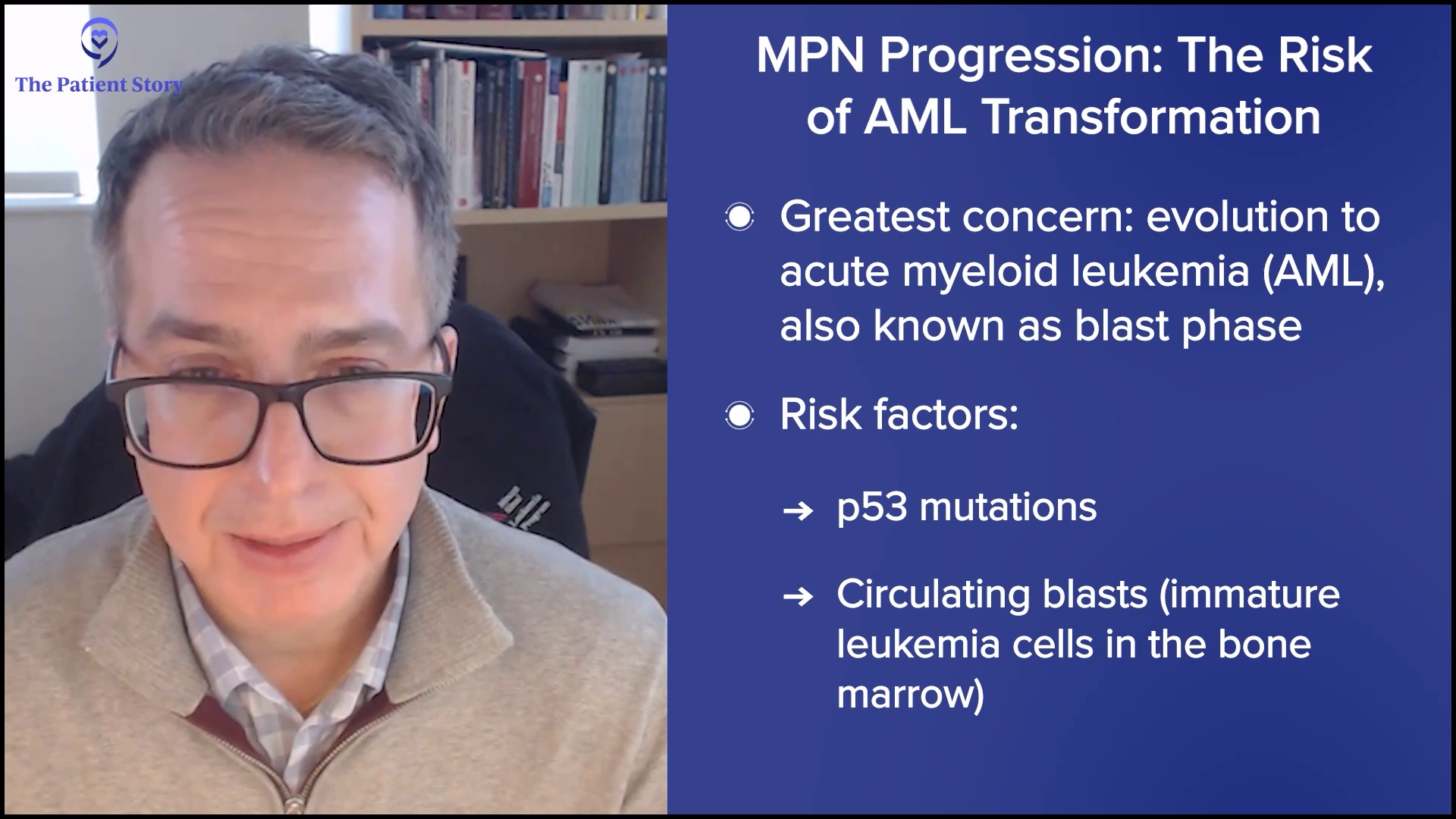
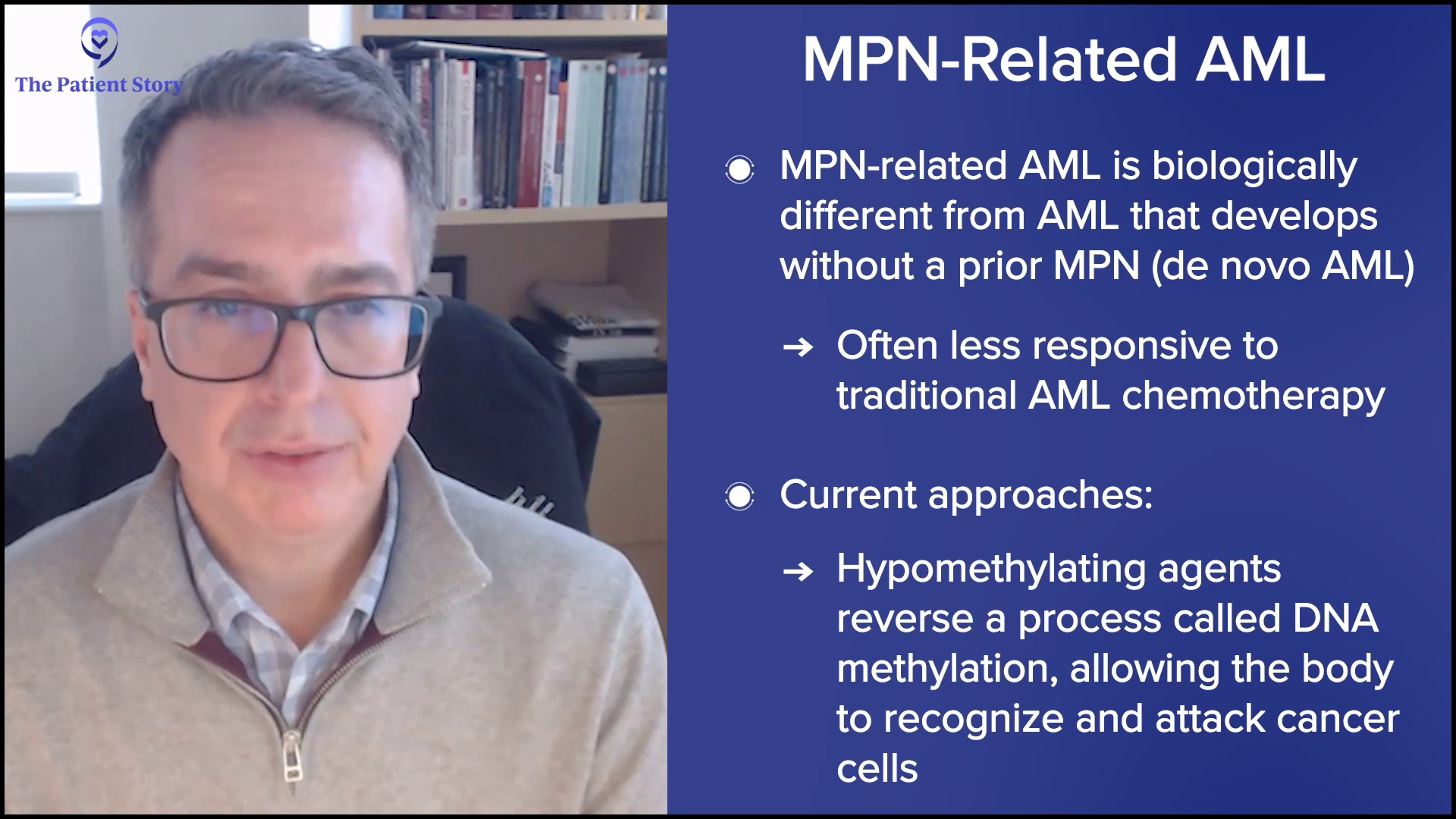
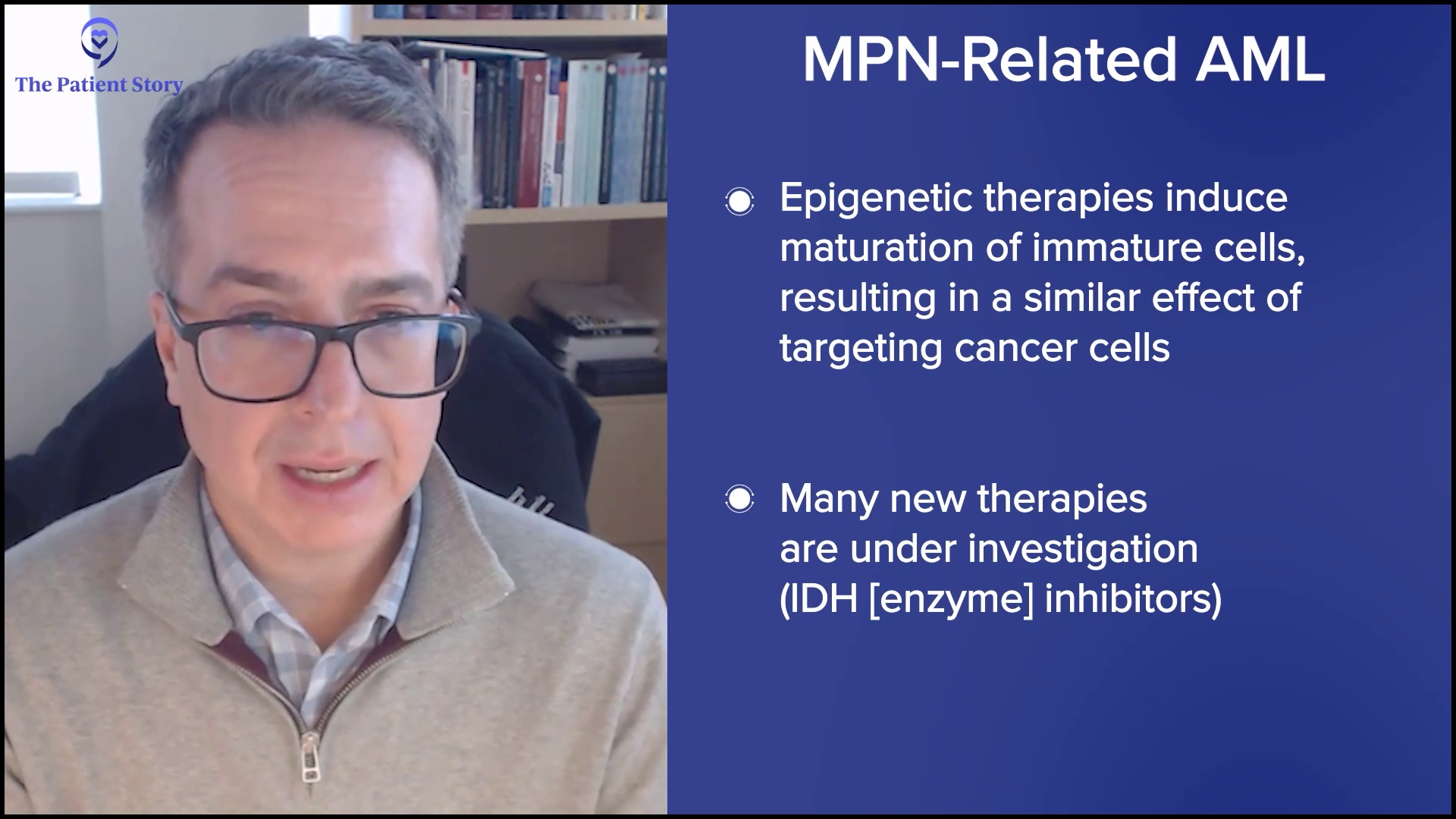
Ruth: Now, let’s talk about progression. It’s a topic nobody wants to face. Or maybe we all want to talk about it, but we don’t want to go there. We know MPNs can progress over time — for example, ET to PV, or for me, PV to MF, and MF can progress to acute myeloid leukemia (AML). That can bring up a lot of emotions, stress, and sleepless nights.
Sometimes we hear that sleeplessness is a side effect of living with an MPN, but I believe it’s not always a biological or molecular response from the disease itself. A lot of it is the stress. Personally, I don’t think about these things during the day, unless I’m doing a webinar like this. But when I close my eyes at night, that’s when everything starts swirling and it keeps me from sleeping.
It’s important to talk about the balance between facing reality, staying present, and being grounded in daily life. We don’t want anxiety or stress to take over, but it’s also important to acknowledge that it’s real. I believe it’s equally important for others to acknowledge that it’s real because sometimes we feel misunderstood. Demetria, are you worried about MPN symptoms coming back?
Demetria: I’m a woman of faith, so I rely heavily on my belief in God. It has anchored me throughout my journey. I don’t believe in crossing over into fear. It’s healthy to have concern and caution about things, but I try not to go too far into the future because that’s when anxiety sometimes can arise, because you’re thinking about the what-ifs. And sometimes the what-ifs never happen.
For me, I rely on my track record that God has always pulled me through. I believe that if something else were to arise, because I’m anchored in that belief that I’m taking care of spiritually, that it’s going to be okay. Sometimes we do have to go through journeys and valleys, but it’s how you respond to those valleys that makes the difference.

Ruth: Nick, what about you? How do you balance living in the moment and not worrying about progression? I also wonder: have you talked with your doctors about this? Because some doctors don’t always ask about mental health or make that connection, while others do.
Nick: I’ve been very open and public about some of the mental health struggles that I went through, especially early on. It was a very shocking and confusing diagnosis. I kept everything inside. I didn’t communicate at all with my wife or my family, and it was deteriorating my mental health.
What helped me was communicating. I communicated a little bit better with my wife. I did that through a documentary that I did, which was life-changing, to be quite honest with you. It wasn’t necessarily about the documentary, but it was about getting involved in the cause. It made me feel like I was doing something to help. You quickly realize that it’s not about you. There’s a greater cause out there. There are other people that you can help by sharing your story and communicating, and that was the turning point for me.
Connect with other patients. It’s so life-changing to speak with someone who is going through something similar and may have a different perspective.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
I did seek professional help. I remember going to the emergency room a couple of times because of the anxiety pains that I had, which I thought then was a heart attack. At that point, everything was all bottled up inside. Again, that was another pivot point to seek professional help.
Both of those things were life-changing. I talk about this all the time: don’t be afraid to communicate. You have to communicate because it is a release point. Get help and also connect with other patients. It’s so life-changing to speak with someone who is going through something similar and may have a different perspective. It is valuable.
Ruth: All of that is thanks to the internet. Obviously, when I was diagnosed 30 years ago, the internet was in its infancy. I didn’t have resources like The Patient Story, which are invaluable for those of us living with any chronic disease, particularly chronic cancer and an MPN.
Primarily my husband and then my mother… With both of them on my team, things that I didn’t say, didn’t know to say, or forgot to say, they would jump right in to say those things for me.
Demetria J.,
Myelofibrosis Patient & MPN Patient Advocate
How Care Partners Can Help You Track Symptoms
Ruth: Let’s move on to the role of care partners in symptom tracking. Do either of you have someone helping you track symptoms or notice changes that you might miss?
Demetria: Primarily my husband and then my mother. My mother has a background in the medical field. Sometimes she would accompany me on my visits and would ask questions that I may not have known to ask because of her background.
When I had my bone marrow transplant, I had to have a caregiver with me. My mother and my husband alternated because I had to go to another city to have the bone marrow transplant, which was two hours away from where we lived. My daughter at the time was 10, so he had to navigate caring for her as well as caring for me.
They were some of my biggest supporters. I remember one doctor visit. I was starting to itch a little bit. I hadn’t said anything to my oncologist about it and he said, “You need to tell him about the itching.” The doctor asks, “What about the itching?” I said, “Yeah, I’m having a little bit of itching.” With both of them on my team, things that I didn’t say, didn’t know to say, or forgot to say, they would jump right in to say those things for me.
Kara, my wife, is my caregiver and I don’t know where I’d be without her… She’ll ask me questions to make sure that I’m being honest about what I’m going to talk to the doctor about.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
Ruth: I know a lot of times people will go to their doctor and say, “No, I’ve been fine.” “Are you fatigued?” “No.” “Have you been tired?” “No.” Then the care partner says, “Well, we’ve had to rest a lot when we’ve gone on our two-mile walks every morning or when going up the stairs.” People in our lives play a role that I think is important. Have there been times when your caregiver noticed something you hadn’t picked up on at all yet?
Nick: Kara, my wife, is my caregiver and I don’t know where I’d be without her. We do a lot of communicating now. I’ve mentioned that I treat the doctor appointments like business meetings, so I will prep with her by going over my notes and what I’m feeling. She’ll ask me questions to make sure that I’m being honest about what I’m going to talk to the doctor about. Then we’ll do a debrief about what the doctor said, what the numbers looked like, and everything in between.
I give her a lot of credit for this. I was having trouble with my vision. I’ve had blurry vision before, but all of a sudden, I was having issues seeing. Everything was coming up blurry and she says, “You’ve been on a certain drug for a while. Do you think that has any effect? Why don’t you bring it up to your doctor?” Lo and behold, it does. There is an impact taking certain drugs with your vision. She’s fantastic. She’s a lot smarter than I am, so I rely on her a lot.
His presence and being willing to sacrifice to go through the journey with me were enough for me.
Demetria J.,
Myelofibrosis Patient & MPN Patient Advocate
Ruth: Demetria, how does your care partner support you emotionally, particularly when you’re dealing with anxiety?
Demetria: Surprisingly, my husband and I have had these kinds of conversations, and he said that the way that I was so calm and remained calm through all of this helped him to stay calm. People look at the husband as the protector, as the one to shoulder the emotional burden to keep the household calm. But he shared that he watched me and how calm I was, which helped him. He felt as if there was nothing he could do to help me in terms of getting through this. We had to work through the process.
There is an emotional and mental component to someone simply being there. It makes you feel confident. It feels like you are seen. He sees me. He sees that I’m going through this. I feel secure because he’s here. He’s not leaving me by myself to go through this journey. Even though he may not have offered any specific emotional or mental help in the traditional sense, his presence and being willing to sacrifice to go through the journey with me were enough for me.
I heard from an MPN doctor that if we ask every person living with MPN if they were willing to participate in a study, 90-something percent would probably say yes.
Ruth Fein Revell,
Myelofibrosis Patient & MPN Patient Advocate
Participating in Clinical Trials
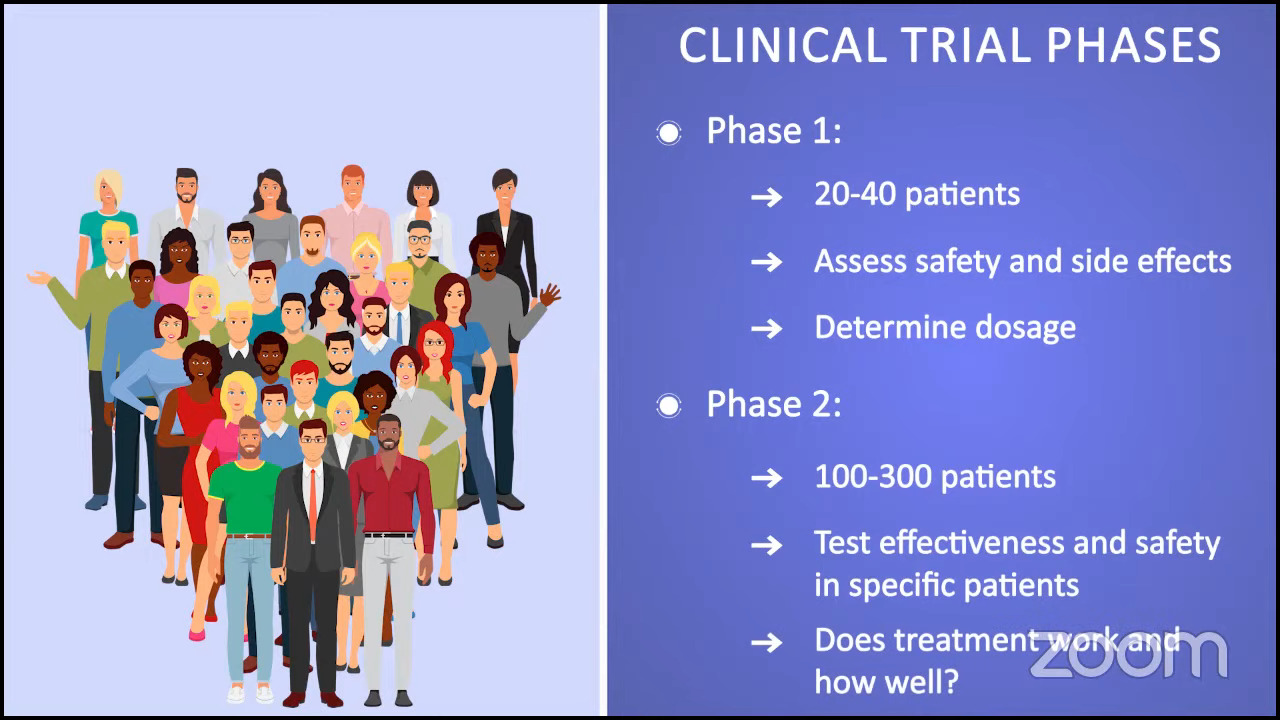
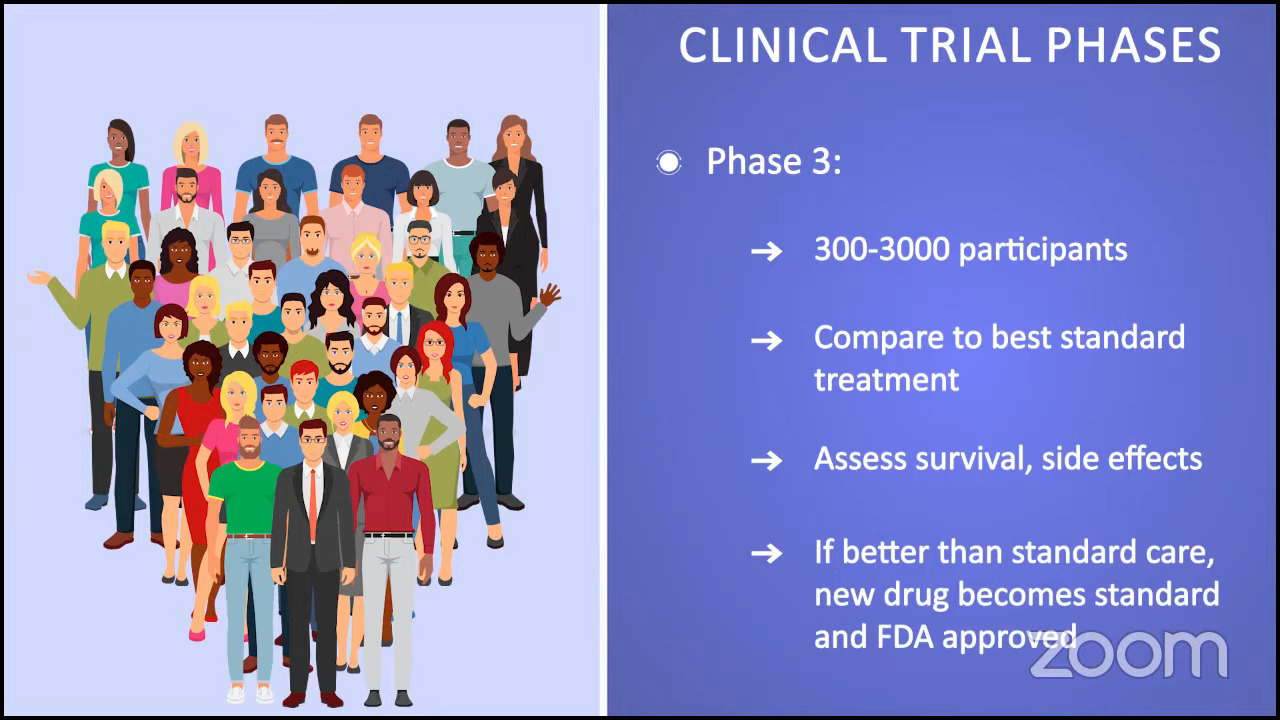
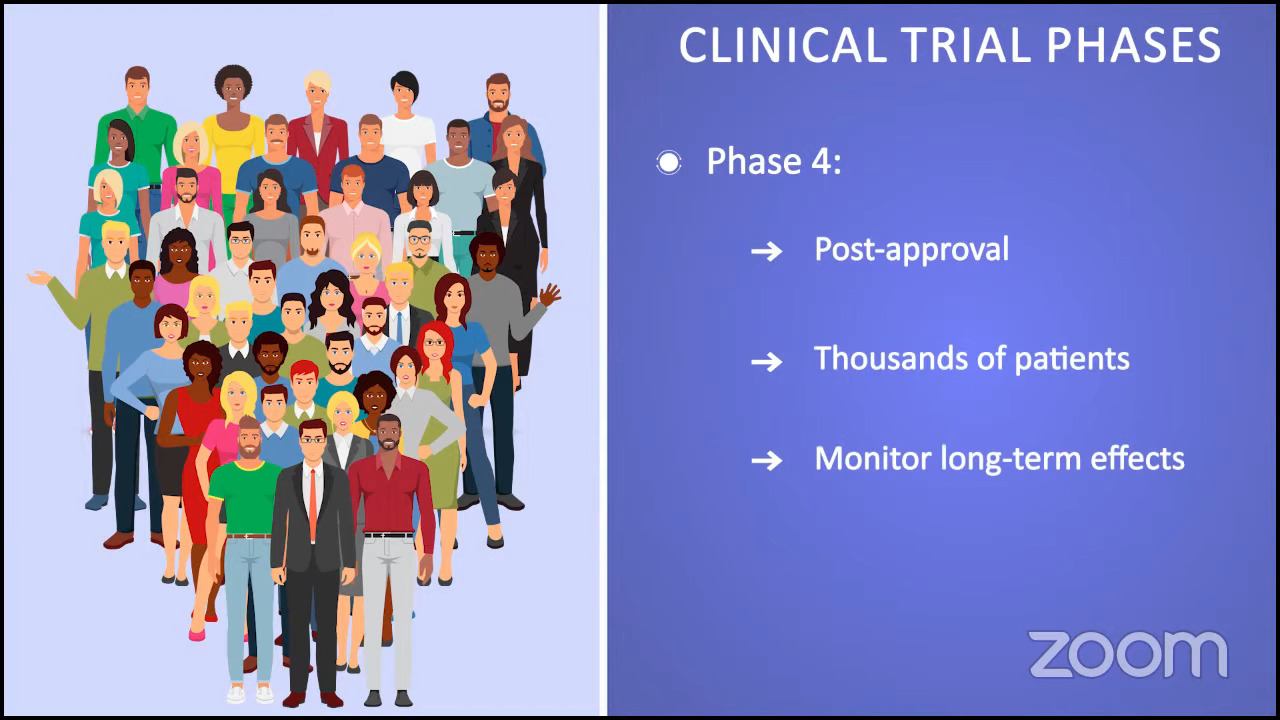
Ruth: We’re moving on to clinical trials and looking ahead, which is important because some of the best drugs for MPNs are still in clinical trials. I like to say that clinical trials are no longer a last resort and I’m a perfect example of that. I’ve been in one for five and a half years, and I’m doing extraordinarily well on that trial.
There are challenges, however. I have to go back and forth 3.5 hours each way to the major academic center where I’m being seen, but I’m having a life-changing result, and to me, that’s worth it. By the same token, not everyone has access to clinical trials, which is difficult, and we acknowledge that.
Has your doctor ever brought up the idea of clinical trials as part of your treatment plan? I heard from an MPN doctor that if we ask every person living with MPN if they were willing to participate in a study, 90-something percent would probably say yes. The only obstacle is that they’re not asked. What about you, Demetria? Have you ever been offered a clinical trial?
Demetria: I haven’t ever been asked. But I have been told about them, especially when I was at Emory, because they do participate in a lot of clinical trials. The conversation was, “If this medication does not work well for you, then we could look at putting you in a clinical trial.”
He heard of a diet study that Dr. Angela Fleischman was doing to take a look at the Mediterranean diet and the impact that it has on MPNs. He thought that would be of interest to me.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
Ruth: What about you, Nick? Is that something you’ve been asked about?
Nick: I will answer this a little bit differently. When I first got diagnosed, I focused on my diet. I tried to take out a lot of the inflammatory foods I was eating and made that a focus of my overall care. My doctor knew that at the time because I talked about that openly with him.
He heard of a diet study that Dr. Angela Fleischman was doing to take a look at the Mediterranean diet and the impact that it has on MPNs. He thought that would be of interest to me, so I participated in it and it was nothing short of fantastic. It validated some of the things that I was doing were correct, but also exposed some of the things that I wasn’t doing, and some of the nutrients and proteins that I wasn’t getting based on my diet, and iron. We don’t need too much iron as MPN patients, obviously, but we need a base level of iron.
It was great and it was educational for me. It’s important for people to know that those kinds of studies are out there, outside of the drug clinical trials that we typically see.
Ruth: Thanks for mentioning that. Dr. Fleischman’s studies are interesting. We can learn a lot, not just from the Mediterranean diet, but from the Mediterranean diet’s influence, if you will — picking and choosing what works for us, but understanding inflammation’s effect on MPNs.
Have an open dialogue throughout your care with your doctor. I never want to feel complacent with where I’m at in my care.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
Considering Clinical Trials in the Future
Ruth: If you were asked, whether it’s today or when things change, what would your care team be able to do to make you feel comfortable exploring a clinical trial?
Nick: For me, it starts before talking about the clinical trial. I feel like you need to have an open dialogue throughout your care with your doctor. I never want to feel complacent with where I’m at in my care, so I’m constantly challenging my doctor. What’s out there? What can we be doing differently, if anything? What’s on the horizon? What if this happens, what do we do? Those are the type of questions that I ask my doctor, maybe every other appointment, and brainstorm.
If you’re having those discussions, it makes that conversation about clinical trials a little bit easier and more comfortable because you’ve already talked about it. Starting with having a regular cadence of talking about the what-ifs and what we could do if things change will help with the conversation about clinical trials and ultimately participating in them.
Ruth: Totally agree. Sometimes we have that conversation long enough that the clinical trial becomes an approved drug, and we don’t have to have that conversation anymore.
If there were newer things out there or that are coming, I want to be in the know and figure out how I can benefit.
Demetria J.,
Myelofibrosis Patient & MPN Patient Advocate
Doing Your Own Research on Clinical Trials
Ruth: Have either of you ever come across a clinical trial in your own research that seemed interesting, but you didn’t know how to move forward or how to talk to your doctors about it?
Nick: I would say I think that’s where the communication part comes in with the patient community. Also, with organizations like The Patient Story, they can get you resources and someone to talk to to further vet that out, and then ultimately maybe find an MPN expert to talk through that in a little bit more detail.
Demetria: Initially, when I was diagnosed with ET, I was very young. I was about 34 when I was diagnosed. I did some research on the medication that she put me on and said to her, “My husband and I may still want to have children, and this medication is not going to fare well if that is the case.”
I researched some new medications and clinical trials that were out. There was one particular doctor whom I mentioned to her that I saw was making headway and introducing new concepts and treatment plans. I asked her, “Is there a way that you can see if these things would work for me?” I didn’t feel like she was willing to do anything other than what she suggested, so that’s how I ended up at Emory.
This is my life. I have to be an advocate for my life. I have a husband and a daughter. I want to live. And if there were newer things out there or that are coming, I want to be in the know and figure out how I can benefit from the newer things that could be better than what she was offering.
All options are on the table. I want to live as long as I possibly can and I will do whatever is necessary to make that happen.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
Ruth: If things change, Nick, are you open to participating in a drug clinical trial, if at some point that becomes the right choice for you?
Nick: Of course. The way I look at my disease and the care is that all options are on the table. I want to live as long as I possibly can and I will do whatever is necessary to make that happen.
Ruth: Thank you for sharing that. I will just add that science is advancing at unprecedented speeds. Thirty years ago, things were so different. Twenty years ago, ten years ago, even five years ago, for that matter. Within the last two or three years, our options have expanded exponentially. The longer we’re well enough to live a good quality of life while we wait for new and better treatments to come along, those decades add up, and that’s a good thing.
Do not think that any symptom is too insignificant. Track everything and let your doctor tell you whether you have to worry about a particular symptom or not.
Nick Napolitano,
Polycythemia Vera Patient & MPN Patient Advocate
Final Words of Advice
Ruth: To move on to final reflections, what advice would you give to someone who was just diagnosed with an MPN about tracking symptoms specifically? Is there anything you’ve learned over time about how to talk to your doctors so they hear what’s going on and see that you’re a knowledgeable patient?
Nick: I would say track it all. Do not think that any symptom is too insignificant. Track everything and let your doctor tell you whether you have to worry about a particular symptom or not.
Be prepared for your doctor’s appointments. Do the research. Communicate how you’re feeling. Be open-minded with treatment options. Be open-minded in getting the care that you need, whether it’s an MPN expert or a mental health expert, if you’re struggling with that. But be open-minded, please.
Demetria: I would say to pay attention. Life is fast. I call this the microwave generation. We do everything fast. We want everything fast. And sometimes when you’re moving as fast as I was, you don’t get a chance to pay attention to what’s going on inside of you. Sometimes when you’re driving on the highway, you’re going so fast that you can’t see anything. But if you’re on the back roads, you have to slow down. You can’t go fast, but you’re able to see the scenery. When you slow down, you can pay attention to see what’s going on.
Sometimes, we need to slow down to pay attention and be intentional about looking at ourselves, looking at our bodies, and evaluating how we feel. How long have I been feeling this way? Don’t discount it as a fluke or an isolated incident. Take it seriously. Take it to your doctors and find out what’s going on so you can live.
Sometimes, we need to slow down to pay attention and be intentional about looking at ourselves, looking at our bodies, and evaluating how we feel.
Demetria J.,
Myelofibrosis Patient & MPN Patient Advocate
Conclusion
Ruth: Thank you so much, Nick and Demetria, for sharing so openly. Your stories are powerful reminders of how even small steps, like tracking symptoms and writing them down, can have a big impact and make a real difference in managing your health.
We’d like to also thank our audience. If you’re living with MPN or caring for someone who is, we hope that this discussion has left you feeling more informed, supported, and empowered, and a little less invisible and misunderstood.
We want to thank our sponsors, Incyte and Karyopharm, for their support of our independent educational program. This allows us to be able to do more content like this one and at no cost to you. The Patient Story always retains full editorial control. While we hope that this is helpful, this is not a substitute for medical advice. Please still consult your own healthcare teams when making your healthcare decisions.
We want to hear from you. Please share your feedback on this discussion and also what you want us to cover next. Thanks so much from The Patient Story.


Thank you to Incyte and Karyopharm Therapeutics for supporting our patient education program. The Patient Story retains full editorial control over all content.
Myeloproliferative Neoplasm Patient Stories
How to Support Someone with Cancer: Karina & Jesse's Myelofibrosis Story
“I underwent a lot of sadness, hardship, and difficulty, and all that entails. But I pressed forward in hope for sure. There was a lot of hope that just kept me going all those years.”
Demetria J., Essential Thrombocythemia (ET) progressing to Myelofibrosis
Symptoms: Extreme fatigue, stomach pain (later identified as due to an enlarged spleen), dizziness, shortness of breath
Treatments: Spleen-shrinking medication, regular blood transfusions, bone marrow transplant
Neal H., Prefibrotic Myelofibrosis
Symptoms: Night sweats, severe itching, abdominal pain, bone pain
Treatment: Tumor necrosis factor blocker, chemotherapy, targeted therapy, testosterone replacement therapy
Andrea S., essential thrombocythemia (ET) progressing to Myelofibrosis
Symptoms: Fatigue, anemia
Treatments: Targeted therapy (JAK inhibitor), blood transfusions, allogeneic stem cell transplant
Demetria J., Essential Thrombocythemia (ET) progressing to Myelofibrosis
Symptoms: Extreme fatigue, stomach pain (later identified as due to an enlarged spleen), dizziness, shortness of breath
Treatments: Spleen-shrinking medication, regular blood transfusions, bone marrow transplant
Neal H., Prefibrotic Myelofibrosis
Symptoms: Night sweats, severe itching, abdominal pain, bone pain
Treatment: Tumor necrosis factor blocker, chemotherapy, targeted therapy, testosterone replacement therapy
Essential Thrombocythemia Treatments (2022)
Dr. Mesa shares the latest on essential thrombocythemia treatment updates to watch out for in 2022.
Taja S., Polycythemia Vera
Symptoms: Chronic fatigue, fainting, stroke-like episodes, elevated hemoglobin, hematocrit, and platelet count
Treatments: Emergency surgery for ruptured cyst & bowel obstruction, chemotherapy, radiation, bone marrow transplant
Jeremy S., Polycythemia Vera
Jeremy Smith and Dr. Angela Fleischman share empowering insights on living well with polycythemia vera, from symptoms to treatment and patient advocacy.
Todd S., Polycythemia Vera
Symptoms: None, discovered during a routine physical that uncovered extremely high blood counts
Treatments: Phlebotomy, aspirin
Nick N., Polycythemia Vera
Symptoms: None, caught at routine physical
Treatments: Phlebotomy, Besremi
Myeloproliferative Neoplasm Resources
Your MPN, Your Journey: How New Discoveries Will Impact Personalized Care
Dr. John Mascarenhas of The Tisch Cancer Institute at Mount Sinai and patient advocate Andrew Schorr share the latest breakthroughs in MPN care.
Srdan Verstovsek, MD, PhD
Role: Director, Clinical Research Center for MPNs at MD Anderson; Section Chief, MPNs; Prof., Dept. of Leukemia
Focus: Myeloproliferative neoplasms (MPN)
Institution: MD Anderson
Myelofibrosis Highlights from ASH 2022
Dr. Serge Verstovsek and Dr. Naveen Pemmaraju discuss cutting-edge treatments and therapies, and combination therapy as a focus in treating myelofibrosis.
Clinical Trials and You: How to Navigate Treatment?
Patient advocate Ruth Fein Revell, experts Dr. Angela Fleischman and Dr. Ruben Mesa, together with clinical trial nurse Melissa Melendez delve into the cutting-edge realm of myelofibrosis clinical trials.
















 Stephanie Chuang, The Patient Story: Hi, there! I’m so glad you could join us. This program is part of
Stephanie Chuang, The Patient Story: Hi, there! I’m so glad you could join us. This program is part of 
 We’re also incredibly lucky to have again Dr. Angela Fleischman from
We’re also incredibly lucky to have again Dr. Angela Fleischman from  Andrew Schorr: I’m Andrew Schorr and I’ve been living with
Andrew Schorr: I’m Andrew Schorr and I’ve been living with