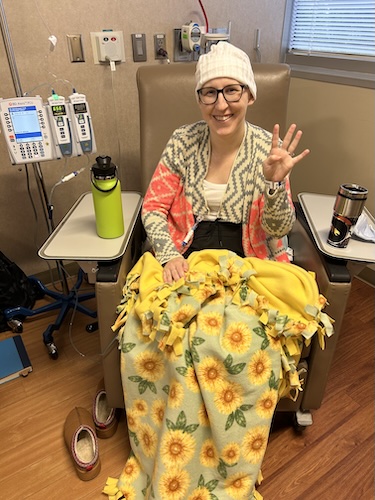Spencer’s Fertility Journey and an Unexpected Ovarian Cancer Diagnosis
Spencer and her husband were in the process of trying to grow their family through fertility treatments. The executive producer, mother, and wife had endured the challenges of IVF before, and in early 2025, a routine baseline screening for a second embryo transfer altered the course of her life. When an unexpected ovarian abnormality appeared, further follow-up and surgery confirmed that she had early-stage ovarian cancer.
Interviewed by: Taylor Scheib
Edited by: Chris Sanchez
The diagnosis of ovarian clear cell carcinoma, a rare and aggressive subtype of ovarian cancer, meant that Spencer had to undergo immediate and life-altering surgery. She underwent a hysterectomy, an oophorectomy, and a salpingectomy to remove her reproductive organs, and she lost the ability to have another child. She openly describes the dual grief of confronting cancer and the end of her personal fertility, navigating both with the support of her husband and community.

Throughout her treatment, which also included chemotherapy, Spencer did her best to maintain normalcy for her young son, relying on family, friends, and a flexible workplace. She shares how complex it is to explain cancer to a toddler, her son’s pragmatic response to the physical changes she underwent, like hair loss, and her increased appreciation for small daily moments that followed her experience. Spencer also discusses the financial and emotional realities of fertility treatment.
Now in remission, Spencer focuses on vigilant monitoring, making full use of her new knowledge of genetic risk factors such as BRCA positivity and Lynch syndrome. Her story highlights the lifesaving potential of routine screening during fertility treatment, the limitations of current ovarian cancer detection strategies, and the power of self-advocacy. For fellow ovarian cancer patients, Spencer underscores the importance of listening to one’s body and asking for support: “You have to be strong, but you don’t have to do it alone.”
Watch Spencer’s video and read through the edited transcript of her interview below to learn more about how:
- Early detection of ovarian clear cell carcinoma can be lifesaving
- The impact of cancer extends beyond treatment to fertility, finances, and emotional health, making insurance and community support critical
- Self-advocacy and attentive listening to one’s own body are crucial for any patient facing a serious health condition
- Asking for and accepting help is an act of strength, not weakness, especially when facing the realities of cancer and intensive treatment
- Spencer’s experience demonstrates personal transformation; she emerged with deeper gratitude and a more present, accepting approach to life
- Name: Spencer L.
- Age at Diagnosis:
- 35
- Diagnosis:
- Ovarian Cancer (Ovarian Clear Cell Carcinoma)
- Staging:
- Stage 1
- Mutation:
- BRCA
- Symptoms:
- None; mass in the ovary discovered during preparation for IVF
- Treatments:
- Surgeries: hysterectomy, oophorectomy, salpingectomy
- Chemotherapy
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make informed treatment decisions.
The views and opinions expressed in this interview do not necessarily reflect those of The Patient Story.
- My name is Spencer
- IVF, fertility challenges, and discovering ovarian cancer
- Processing the word “oncologist” and the fear before surgery
- The agony of waiting and realizing that I had been walking around with cancer
- Grief, embryos, and not being able to carry another child
- Motherhood, perspective, and how my son grounded me
- Navigating marriage, communication, and shared loss
- The financial reality of IVF and fertility care
- Infertility, guilt, and a late discovery of silent endometriosis
- Choosing to work through chemotherapy
- Hair loss, wigs, and my son’s reaction
- Balancing work, income, and intense chemo
- Telling coworkers about cancer and being seen
- Ringing the bell and post-chemo scans
- BRCA, Lynch syndrome, and lifelong surveillance
- IVF as a life-saver
- Living in survivorship and learning to let go
- Asking for help, community support, and redefining strength
My name is Spencer
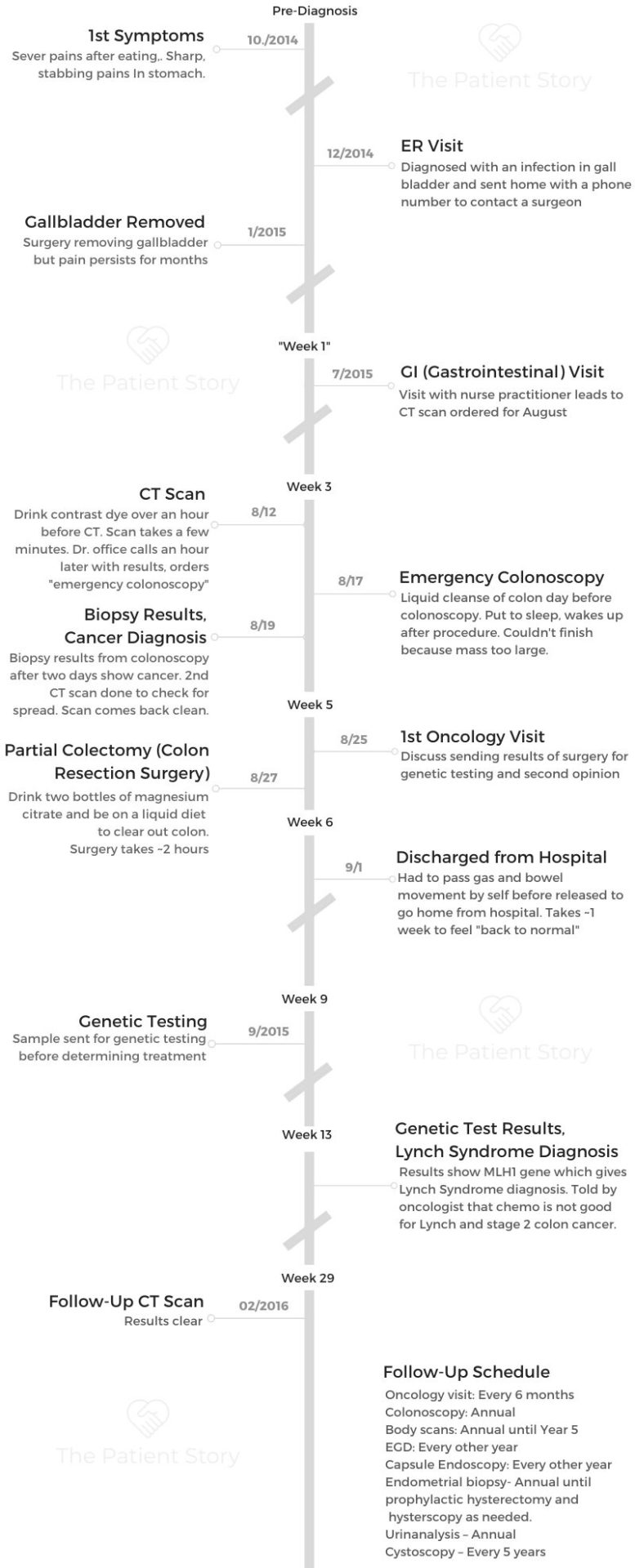
I was diagnosed with ovarian cancer in April of 2025. It was stage 1 clear cell carcinoma, to be exact. I am from Charlotte, North Carolina, originally from Las Vegas, but I have lived in Charlotte for 11 years now.
I am a mother and a wife, and those are the two titles I am most proud of. My son is two and a half. I have been married for just over six years now, but my husband and I have been together for a little over 11 years.
I love to read. I am a part of the Junior League of Charlotte and have held some good roles there. I took a little bit of a step back after I was the Executive Vice President. I am also a part of the Zeta Tau Alpha alumni group here in Charlotte. I was a Zeta throughout college.
I am an executive producer at WCNC Charlotte, so I work with our reporters, our producers, and everyone in the building to tell the news and what is going on in Charlotte.
I am a daughter, a sister, a niece, and a cousin. I love my family.
IVF, fertility challenges, and discovering ovarian cancer
We did IVF to have our son. That was a long process. We had talked about whether we were going to have another child. Initially, my husband and I both wanted three kids, but life took turns, and it took longer, so we decided maybe we would have two. I was a little bit on the fence, and I think I was just scared. We had gone through some miscarriages, and IVF is tough, so it was not an easy process to get pregnant. Just knowing I would have to go through that again was hard. But we ultimately decided we would try.
In January of 2025, I went to my IVF clinic to get some of the first baseline testing done, just to make sure we were good to do another embryo transfer. They took some blood work and looked at my lining. They said everything was great, and to come back in a month and start my meds. A couple of weeks from that appointment, I was supposed to come back in a month, and we would be ready to transfer.
I came back almost a month to the day later in February, and they told me most things looked good, but my right ovary had doubled in size in a month. They did not really know what was going on and told me to come back in two days to see the actual doctor so he could get a better look at it.
I came back in two days. He said he did not really know what was going on and that it was weird that I was not in pain. He said he would think I should be in pain, but I was not. He sent me straight to an oncologist. That was on a Thursday. I saw the oncologist on Monday.
She had seen the ultrasound they sent over and thought maybe it was just a mass, especially because I was not having any pain. I am young, so there is that thought that cancer does not come this young. We know that it does, but I was not in any pain, and I did not really have any symptoms. She took some blood work, and one of my cancer markers was high, but she said that could be high from other things going on in my body. I still left that appointment hopeful.
We scheduled a surgery because she said if I wanted to get pregnant, I should not have this mass or cyst in my body. We agreed to plan to get it removed, no matter what. That was February 24th. My surgery was scheduled for April 2nd.
We had a conversation about what to do if it were cancer. She said they would test the tumor after they removed it. They would test the mass for cancer, and if it came back positive, did we want them to take everything, or did we want them just to stitch me back up so I could decide later? I said that if it was really bad news, if it was high grade and highly aggressive, to just take everything. My main thing was that I wanted to be alive and here. That was what mattered.
I went in for the surgery and woke up still groggy. My husband had to tell me that it was cancer and highly aggressive. They had removed everything: my uterus, my ovaries, my tubes, everything. In a span of six weeks, I went from thinking I might have a second child to being told I was never going to carry a child again. It was hard, but I am glad that I am here, and that was my number one thing.
I feel crazy saying that I kept battling between whether I wanted a second kid or not, and I kept thinking, “Send me a sign, send me a sign.” I remember thinking, “I did not want this big of a sign, but I guess that is my answer.”
Processing the word “oncologist” and the fear before surgery
I remember being told I was being referred to an oncologist and just saying, “Okay, okay.” I do not think in my mind, at that moment, I fully recognized what an oncologist was. Then I got this call: “Hey, this is Levine Cancer Institute with the oncology department,” and that was when it hit me. I thought, “Oh my God, oncology is cancer.” I asked the woman on the phone why she was calling and said I had just been told to be there, and asked if she could tell me what was going on. She said she was just the scheduler and was sorry. I apologized to her because it was not her fault, but I felt so thrown.
I was so thrown by going from “Okay, I am going to have this kid,” to “No, now you are not,” and I was not processing it well. As bad as it sounds, that first initial moment was the hardest part for me. I was more scared then than I was when I woke up from surgery and was told I had cancer. I think it was because that was the initial part. After that, I had about five weeks to stew.
Once I learned about the symptoms, I started being more in tune with my body. I think the symptoms started coming after I heard about them. I was losing weight, I got fuller more quickly, and I had some bloating. I started thinking this could actually be cancer. I had five weeks to stew with that possibility and know it was real.
It is crazy because at that first oncologist appointment, she said surgery was probably in about five weeks. I remember asking her if it was five weeks because she did not think it was cancer, and if it would be sooner if she thought it was cancer. She said no, they were just really booked up. I thought that was crazy. Maybe if they really thought it was stage 3 or 4 cancer, things would have moved quicker, but I kept thinking, “Thankfully it was stage one, because I would have wondered the whole time whether those five weeks mattered.”
The agony of waiting and realizing that I had been walking around with cancer
The waiting was awful. People say the waiting is the worst part, and it really is.
You cannot do anything. You are just waiting and sitting in your thoughts, and that is not healthy.
I did not feel like I had been experiencing symptoms before all this, so realizing I had been walking around with cancer in my body was terrifying.
Once I knew there was something there, I became more aware of every feeling and change. I started connecting potential symptoms in a way I never would have before.
The more I learned, the more I saw how easy it would have been to dismiss what was happening as something minor.
Grief, embryos, and not being able to carry another child
Some days, I do not know if it has fully hit me that I will never carry another child. We still have embryos, so we could have a biological child, but I would not be the one to carry the pregnancy. I remember getting a bill from our fertility clinic saying we had to pay for the next year of storage for our embryos. I had that thought of whether we should do it or not. Everyone around me said not to think about it yet, but I had to think about it because I had to decide whether to pay for the storage or not.
We chose to pay for it because I could not fully think it through, but I did not want to totally give up yet. We are still going through that. I have looked into surrogates, but they are so expensive. People say we can just do that, but it is not that simple. We have some IVF and fertility insurance, but it does not cover nearly as much as a surrogate would cost.
I still go up and down with it. I think about how I was asking for a sign, and this is the sign, but it is a brutal one. I do not know if I have fully dealt with it yet because I have been trying to deal with the cancer and stay alive. I have been trying to focus on being here. If I am being honest, I do not think I have fully processed the loss of carrying another child.
Motherhood, perspective, and how my son grounded me
I say that every pregnancy is beautiful and everyone is appreciative of being pregnant, but people who go through IVF and have had losses appreciate it differently. I feel like I appreciate having a child a little bit more.
I feel terrible saying that because I know everyone loves their kids, but there is a different layer of gratitude when you have fought so hard to get there.
The other day I woke up and work was already go, go, go. People were calling out, and I was thinking, “That is more that I have to do now.”
My son was awake, and I went in to get him out of his crib. He said he wanted to read some books. We read a couple of books, and he was just so joyous about the books and each page. I remember thinking, “This is what matters. That’s all that matters.”
I am sure I appreciated him before, but now I hold on to him even tighter. He has been the daily reminder of why I wanted to live and what I was fighting for.
Navigating marriage, communication, and shared loss
Neither my husband nor I is very emotional. I do not know if that is good or bad.
I remember talking to him either the night before surgery or the week before and saying that if it was cancer, what did he want me to do. I asked if he wanted me to get stitched back up so we could figure it out later, or if he wanted them to take everything. He kept saying it was up to me, but I told him that if they had to take everything, it would impact him, too. When it comes to another child, that is not just about me.
He told me he wanted me to be alive. If they needed to take everything, they should take everything. I said, “Okay.”
He has always been a partner who is very 50/50 with things, and even more so now. He has been great with our son, the house, groceries, and cooking. He is the better cook, so he has already cooked more than I did, but he stepped up even more.
We have tried to live life as much as we can. I was lucky I did not have super bad side effects, but I still had to be vulnerable at times and ask for help, which I am not good at doing.
I also had to remind myself that I was not angry at people; I was angry at the situation. That helped me keep perspective when my emotions were high.
The financial reality of IVF and fertility care
The financial side of IVF and alternative ways of having a child is a lot. I am lucky that both my husband and I had IVF benefits through our jobs. We still had to meet our deductible, which is high and feels crazy, and we did take out a small loan for part of it. Thankfully, we have paid that off now, but we did it just so we could make it happen.
So much of IVF coverage is paying up front, whereas with other medical bills, you can often get on a payment plan or receive your bill later and deal with it over time. I was lucky to work for a place that has IVF benefits, so we could pursue that path.
I am in Facebook groups full of women who ask how others are doing this with no coverage. It is super unfortunate. There is a lot of push to get more benefits covered because fertility treatment is more common than people realize. More and more women and couples are going through this, and not just heterosexual couples; LGBTQ couples need fertility support to build their families, too.
It should be something more affordable and accessible.
Infertility, guilt, and a late discovery of silent endometriosis
I remember feeling like there was something wrong with me. My husband felt defeated, too, when we finally moved into the IVF world. He felt like he could not provide for his wife to have a child. We both got tested and were told nothing was clearly wrong, which made it harder because we did not understand why it was not working.
On the cancer side of things, I now see some things that were probably making it harder. During my surgery, they told me I had some endometriosis. I was shocked because I had gone through all that IVF work, and no one ever found it. My doctor said it is hard to diagnose, and you basically have to see it, which requires being cut open. Again, I was not having symptoms, and I had heard people talk about silent endometriosis and assumed I did not have it. Maybe I did.
Looking back, I think that could have been part of what made it harder for me to get pregnant and then harder to continue to carry a child once I did get pregnant.
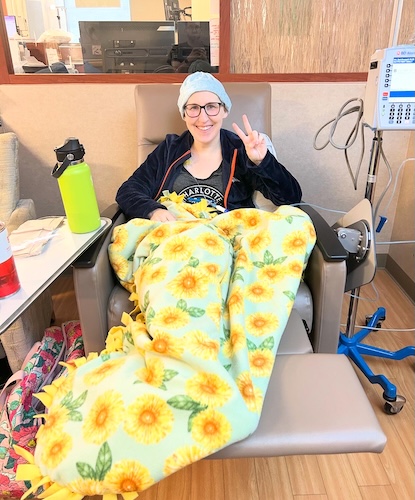
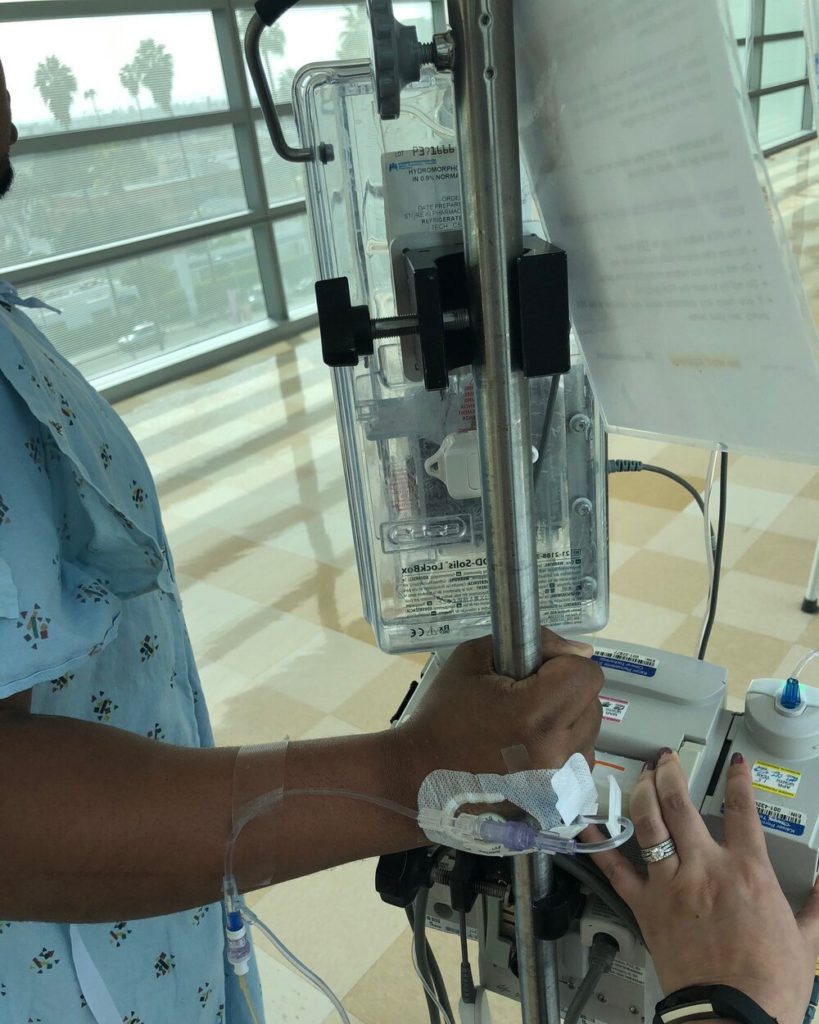
Choosing to work through chemotherapy
I coped with treatment by trying to live as normal a life as possible. I used no PTO days for the chemo infusions themselves. Round one of chemo was the day after my son’s second birthday. I made sure to have his birthday party and enjoy the weekend. Then we went into round one. My family was in town for my son’s birthday, and I felt pretty good overall. We had plans and went out around the town. I went to a friend’s child’s fourth birthday party. I worked and felt okay.
I had that week off work anyway because I had taken it months earlier for my son’s birthday and family visiting, so that timing worked out. After round two, we went to Florida for the 4th of July. Round three was when things started to hit a little harder. I think round three was the first time I had to call out of work for a day because work was crazy and I was doing too much. I realized that was not great.
By round four, I decided I would work from home a couple of days a week after chemo, and that was much better. I did not do that for round five because we were so busy, and then I had to call out again. By round six, I knew I definitely needed to work from home after treatment.
I had a great job with flexibility and great bosses. That made such a difference. I tried to balance rest with not just lying in bed, because lying around made my body hurt worse. I really wanted my son not to know too much. His being two was, in a way, a good age where he did not really understand, which was nice. At the same time, I would have to tell him I could not play right then and needed to lie in bed.
He was also the one who got me out of bed every day and kept things normal. I was especially worried about him seeing me lose my hair. That felt like it would be really hard for him.
Hair loss, wigs, and my son’s reaction
We shaved my head one day and made it a shaving party with some friends, but we did it during my son’s nap because I thought it would be too hard for him to see. I had some wigs ready. We did the shave on a Monday, and I did not show him my bald head until Thursday. I was really nervous.
We filmed his reaction. It was just normal to him. He thought it was funny. He wanted to take the wig off and put it back on, so you can see us playing with it in the video. Some days he wanted to wear the wig himself, so I put it on him a couple of times. It has been normal to him. I think once my hair grows out, he will be confused about why it does not come off anymore.
I told my husband I hope our son starts going to him and asking him to take his hair off. Thankfully, he has not tried that with anyone else. I think he knows it is just a “Mommy thing.”
We will see what happens as my hair grows back.
Balancing work, income, and intense chemo
My plan was always to work through chemo because we needed my income, and also because I wanted to stay connected to something that felt normal. I really love what I do, and I knew that if I could work through treatment, it would give me a sense of normalcy. When I was out on maternity leave for 12 weeks, I loved the time with my son, but I was excited to get back to work because it is a big part of who I am. It makes me a better person and a better mother.
Chemo days themselves were five to six hours, so I took those days off. My chemo days were on Fridays because my husband was off on Fridays and could come with me. I was usually back at work by Monday and, for the most part, felt pretty good through most of the rounds, especially at the beginning.
Telling coworkers about cancer and being seen
I think my boss checked in the day after my diagnosis, or I called him the next day, and I told him what was going on. I told him he could share with people if he wanted to, so I would not have to be the one to tell everyone. He said he would, and I was grateful he shared with the other managers.
I am not an emotional person, and I did not mind people knowing, but I knew people would feel bad for me. That was one of the hardest parts: having to share news that would make other people feel bad. I knew I had to tell people, but I did not want to.
People at work already knew I was going to be gone for two weeks for surgery because I had told them I had a mass and needed surgery. That part was not a surprise. After I got my diagnosis, I told my direct team and some people I was close to. Then I realized everyone would need to know eventually, and I did not want to be texting every single person.
A couple of days before I went back to work, I made a long social media post about everything. When I went back, some people came up and talked to me, and others just commented online or checked in that way. When I finished chemo and rang the bell, one of my friends who works with me was there. She sent the video to the station, and they played it on the newscast that day.
When I came back on Monday, someone said they had no idea. I explained that was why some days I had turquoise hair or pink hair, or short hair, or long hair, or a hat where you could tell I had no hair underneath. It was nice that people did not necessarily see me as that different or treat me like I was fragile. At the same time, it was still hard because I did not want to make people feel bad, but I also needed them to know what was going on.
Ringing the bell and post-chemo scans
Ringing the bell was complicated emotionally. You ring the bell, but you still have to do a scan after chemo. I am very superstitious and believe in jinxes. I worried that if I rang the bell and then had a scan in a month that was not clear, I would feel stupid for celebrating.
I tried to remind myself that my scan before chemo had been clear, so it really should not come back during chemo. I told myself I should be okay and that I would be glad I rang it. I was really happy I did. My husband brought my son, and a couple of close friends who had been checking in on me and supporting me came as well. I brought cake or cupcakes, balloons, and flowers. It made the day feel celebratory and special.
My last round of chemo was September 19th. My scan was on October 31st, Halloween. I was a rare case in the sense that I had stage 1 ovarian cancer, which is not common to catch that early. After surgery, I did a scan before I even started chemo, and it was clear. They believed they had gotten everything with surgery, but cancer cells are tiny, and you do not always see everything. They still wanted to do chemo to kill any remaining cells. I agreed.
I expected my post-chemo scan to be clear, but I was still nervous. I had five weeks between chemo and the scan. Cancer usually does not come back that fast, but so much of my case had been rare that I was worried I would get the bad kind of rare. It was all clear. Everything looked good.
Right now, I see my doctor every three months for blood work. The plan is to monitor my tumor markers that way. She does not want to scan me over and over unless my blood work shows something concerning or I have symptoms. If I get to the six-month or one-year mark and feel nervous, she said we can scan then.
BRCA, Lynch syndrome, and lifelong surveillance
During all of this, I found out that I am BRCA-positive and have something called Lynch syndrome.
BRCA increases the risk of breast cancer and ovarian cancer. Lynch syndrome increases the risk of ovarian cancer, colon cancer, skin cancer, and a handful of other cancers.
Because of this, I now have a yearly dermatologist appointment so they can check my skin. I do breast MRIs and mammograms, rotating every six months. I have already had both this year, and they were clear. I will start colonoscopies next year.
It will be a lot of doctor’s appointments, but hopefully all of them will be preventative and allow us to catch anything early.
IVF as a life-saver
I say that doing IVF saved my life. My husband and I had a conversation about whether to have another child on our anniversary in September 2024. We decided we would start trying again in the new year. I think about how bad things could have gotten if I had not gone when I did.
I was not having symptoms at that time. Even when I did start noticing symptoms, it was only because I knew what they were and was looking for them. Otherwise, I would have thought they were just PMS or my body being weird. The symptoms were not super painful or obvious. That is what is so weird and scary about cancer: the symptoms can be vague and easily attributed to something else.
I think about the timing of going in for IVF and how, during a transvaginal ultrasound, they caught my enlarged ovary. There is no standard screening test for ovarian cancer. We do mammograms for breast cancer, but for ovarian cancer, we do not have a routine screening that everyone gets. There is a blood marker test, but that is usually done once there are already concerns.
I was lucky that it was seen incidentally on that ultrasound. It makes me wonder why we do not do transvaginal ultrasounds yearly for women, or at least for those with risk factors. We should be doing more to prevent things. Breast cancer is more common, but often caught earlier and is not as deadly as ovarian cancer. Ovarian cancer is often caught late, and that is why it is so deadly.
Living in survivorship and learning to let go
Each bit of good news felt like a physical weight lifting off my shoulders. People talk about that metaphorically, but I actually felt my shoulders drop after each positive update. I am a nervous patient in general. Before every chemo round, they tested my blood to make sure my body was recovering and checked my tumor markers. I would be so nervous waiting to see if my marker had risen.
They would take my blood pressure and tell me it was a little high. I would say it was because I was sitting there waiting for results. The moment they told me things were okay, and I could move on to the next round, my blood pressure would come down. After my post-chemo scan came back clear, my blood pressure was totally normal. There was nothing to be anxious about in that moment.
Each bit of good news made me feel like I could make a plan for next year, next week, and next month. I am very much a planner, so not being able to plan during treatment was tough. I would think, “Okay, three days after chemo, I might be okay to do something, but five days after, maybe not.” I had to schedule my life around that.
Now I am learning to let things go in a new way. My son had picture day recently. I had an outfit picked out for him, and he said no; he wanted to wear his football shirt. Before all this, I would have insisted on the outfit I chose. Instead, I said okay, he could wear the football shirt. I thought, “What does it matter? You might not even order the photos. This is who he is right now; he loves sports and balls, and that is what this photo will show.”
We took our Christmas pictures the week before. I had lost all my eyelashes at the very end of chemo. I am really low maintenance and have never worn fake eyelashes. I suddenly felt like I needed eyelashes for the photos. I bought lashes and glue and had someone at work show me how to use them. Then I lost the glue and could not find it in time. I was so upset at first, but then I had to tell myself this is my life right now, and it is okay. People are not going to notice as much as I do. I remind myself to have fun and to let go of things that do not truly matter.
This time of year, with the holidays, is full of plans, and I love that. I also have to remind myself to schedule rest because if I do not, I will go, go, go, my body will hurt, and it will be mad at me later. I am trying to balance what is realistic for me to do with permitting myself to rest.
Asking for help, community support, and redefining strength
I would tell anyone going through something similar to advocate for themselves and listen to their body. Ask for help when you need it, because you are going to have to be so strong. I found so many people wanted to help me, even if it just meant talking.
At first, I did not know how I needed help. I would say we were fine. People kept asking, and eventually I told them to let me get through that round, and then I would tell them what I needed. So many people want to help and be there for you. They feel bad if they cannot, so it is important to find ways you truly do need help and be specific about it.
I realized dinner was really hard. I often did not have the energy to cook. So being able to say, “Dinners are hard. I do not have the energy. Can you send this?” made a big difference. Whether you want something specific or are okay with anything, having people take that off your plate is huge.
It is okay to take each day in stride and to accept that you are not going to be exactly who you were before. In a way, this experience changes you for the better because you see life in such a different light.

Inspired by Spencer's story?
Share your story, too!
More Clear Cell Ovarian Cancer Stories
Sara I., High-Grade Serous & Clear Cell Carcinoma, Stage 3A
Symptoms: Random sharp pains, unrelated scan showed ovarian cyst
Treatments: Debulking surgery, chemotherapy (carboplatin & paclitaxel), PARP inhibitors (clinical trial)
...
Kim C., Ovarian Cancer (Clear Cell Carcinoma), Stage 2B
Symptoms: Coughing, incontinence, severe bloating, nighttime pain, hard lump on right side
Treatments: Surgeries (radical hysterectomy, lymphadenectomy), chemotherapy, radiation therapy, immunotherapy
...
Kim C., Clear Cell Ovarian Cancer, Stage 4 (Metastatic)
Symptoms: Coughing, incontinence, severe bloating, nighttime pain, hard lump on right side, appearance of rash on chest
Treatments: Surgeries (radical hysterectomy, lymphadenectomy), chemotherapy, radiation therapy, immunotherapy, clinical trial
...
Spencer L., Ovarian Cancer (Ovarian Clear Cell Carcinoma), Stage 1
Symptoms: Mass in the ovary discovered during preparation for IVF
Treatments: Surgeries (hysterectomy, oophorectomy, salpingectomy), chemotherapy
...