Shahzad’s Stage 4 Refractory DLBCL Non-Hodgkin’s Lymphoma Story

Here’s a story of successful CAR T-cell therapy response for Shahzad, who’d been diagnosed with stage 4 Non-Hodgkin’s Lymphoma, subtype diffuse large B-cell, and given 3 months to live.
That’s when his wife, Nicole, championed for him to get this new line of treatment. He was actually the first to undergo a commercial CAR-T drug at Stanford Medical Center. Here’s the incredible story as told by Nicole.
This interview has been edited for clarity. This is not medical advice. Please consult with your healthcare provider for treatment decisions.
1st-Line Treatment
Initial diagnosis
Shahzad was stage 4 with non-Hodgkin’s lymphoma diffuse large B-cell by the time he needed CAR T.
How did 1st-line treatment end?
Due to an initial misdiagnosis of mantle cell blastoid, Shahzad underwent R+B (rituximab and bendamustine) and then R-ICE (rituximab, ifosfamide, carboplatin, etoposide).
When he was properly diagnosed, he underwent dose-adjusted R-EPOCH chemotherapy, but miscommunication led to 3 cycles of that chemo instead of 6.
He relapsed, and we were told by doctors that there was nothing that could be done. He was given 3 months to live.
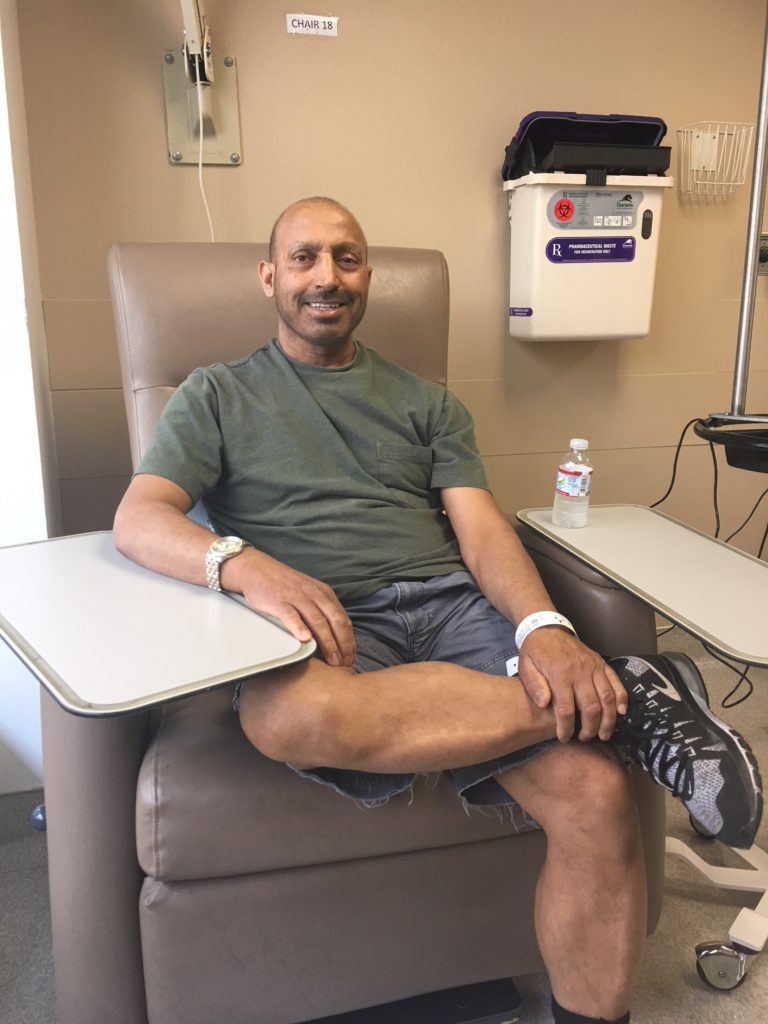
How did you know about CAR T-cell therapy?
My son and I attended the Leukemia and Lymphoma Society Conference in San Francisco. The breakout group we chose was for diffuse large B-cell.
The presenter was Dr. Babis Andreadis of UCSF. He could have chosen any subject to present. Lucky for us, he chose CAR T-cell therapy.
I remember the room had about 30 people, and there was silence, really no reaction. I couldn’t understand why. The material was presented in layman’s terms, easy to understand. I’m not sure why no one seemed interested.
I was blown away! This is the solution for my husband. I just had that gut feeling, that moment of light and insight.
CAR T-Cell Therapy Approval
The start of research
He said it was still in clinical trials and fairly new, so it would be another 2 years before the Food and Drug Administration (FDA) would approve it. 2 years is a very long time to a stage 4 cancer patient.
From that moment on, I began researching. I taught myself about clinical trials and what to look for, inclusions and exclusions.
Insurance
Insurance didn’t seem too committed to covering it, so I had to search for financial assistance, which was difficult because our 2016 income disqualified us on paper.
I continued to follow the progress. Kymriah, the Novartis CAR T-cell therapy for pediatrics acute lymphoblastic leukemia (ALL), was approved in August 2017, several months earlier than expected.
Then an independent committee voted unanimously to recommend approval to the FDA for Yescarta (commercial CAR T-cell drug).
The FDA approved Yescarta on October 18th. On October 19, Shahzad had a biopsy because doctors could not agree on the PET scan images. I remember feeling conflicted. Of course I didn’t want him to have disease.
Or did I? In order to qualify for Yescarta, you must have some disease for the T-cells to work. I felt relieved because I knew that regardless of the outcome, we would be okay.
I never wanted the stem cell transplant. My gut just felt it was not in his best interest. The following week when the doctor told me, “The biopsy was positive. He is now considered relapsed and refractory, disqualified for stem cell transplant, out of options, and has 3 months to live.”
I piped in with, “What about CAR T-cell therapy?” She said that he’s an excellent candidate for CAR T-cell, but her facility won’t be ready until February, and he doesn’t have until February.
That is when I turned to Kite to find out who nearby is ready. I wrote to Dr. Miklos at Stanford. He said he had over a hundred referrals waiting, but given my husband’s timeline, he put Shahzad at the top of the list.

How did you get him approved for CAR T-cell therapy?
Once I knew that Stanford was a viable option, I began a campaign strategy in my head with 2 objectives:
- I had to gather what I thought would be compelling information for the insurance.
- I had to find a way to appeal on a personal level.
1. Compelling Information: I obtained Letter of Statement from 3 separate oncologists, all of whom had evaluated Shahzad, stating that he was indeed a qualified candidate for this treatment, that he only had 3 months to live, and that this was his only option for survival.
I printed off the Yescarta brochure so there was clear understanding of what the therapy involved. I obtained literature that demonstrated a cost savings of Yescarta versus an allogeneic stem cell transplant with graft-versus-host disease (GvHD) lifelong side effects.
Now they can’t argue the price.
2. Personal Appeal: I called our case manager, whom I had never seen, only spoken to over the phone. I asked for a face-to-face meeting because what I needed to discuss was too important to be discussed over the phone. She agreed.
I prepared a simple timeline from symptoms, diagnosis, treatments, to now. I reminded her where and what he’s been through, where he stands now (with 3 months to live), and [that] there is one option left. Can we do this?
She said yes to Yescarta.
Back-up plan
Had she said no, I was prepared to make this public. I had obtained the names and phone numbers and email of each of the board of trustees. I was going to make flyers and postcards and social media pleas to flood their voicemails and emails!
I was going to send them each a party invitation, asking them if they had the chance to save someone’s life, would they? I was going to contact local media and stage picket lines. I was not taking no for an answer.
I was going to fight, fight, fight, and even if they didn’t change their mind, the negative publicity does not do a local union good. Thankfully, Stanford’s finance department and our insurance were able to come to a financial agreement very quickly.
There are different “exceptions” for different kinds of CAR T-cell therapy drugs
CAR T-cell is the therapy, immunotherapy, which targets CD19 protein. Shahzad had that, but he also was positive for CD5.
Clinical trials have a huge list of inclusions, what is required to be accepted into the trial, as well as a huge list of exclusions, which disqualify you from a trial.
During the clinical trial phase, since Shahzad was predominantly CD19 positive qualified him for the trial. The fact that he was also CD5 positive excluded him from trial, because the trial was only targeting CD19. The CD5 was an unknown.
However, when the FDA approved Kite’s Yescarta as a third-line therapy, they did not make CD5 positive an exclusion. That means a patient mostly CD19 positive, even with a CD5 positive, made him qualified to accept the commercial brand.
Questions for doctors about CAR T-cell therapy after he was approved
I had been following CAR T-cell for the year. The only question I had to the doctors were:
- If CAR T fails, how will we know if CD5 was a factor? A blood test would reveal what protein(s) remain.
- I know there was a death during this trial. What caused it? Is it a risk, or what steps have you taken to prevent it?
The death was caused by cerebral edema, which they did not expect as a severe adverse side affect. Now they know how to recognize the signs and bring in the neurologist immediately, so it will never get to that stage again.
What were the biggest hurdles?
At that point, the insurance approved the consultation, which was done in November. That bought a little more time for the rest of the contract to be negotiated.
One site gave me a little trouble about getting records sent over quickly, but I just went up the chain until I got someone compassionate and empowered to help me.
There was an error made on the initial contract, which I found out about as I stayed on top of the timeline. I knew when something fell through the cracks and just helped to keep it moving along.
Once the contract was finalized, Stanford enrolled Shahzad with [the pharmaceutical company], and it makes the schedule based on lab production. We were lucky that it was so soon after FDA approval and not many sites were certified, so the calendar was pretty open for us.
As a result, Shahzad was the first patient to be treated with Yescarta at Stanford. Compared to everything else, the hurdles were really minor.
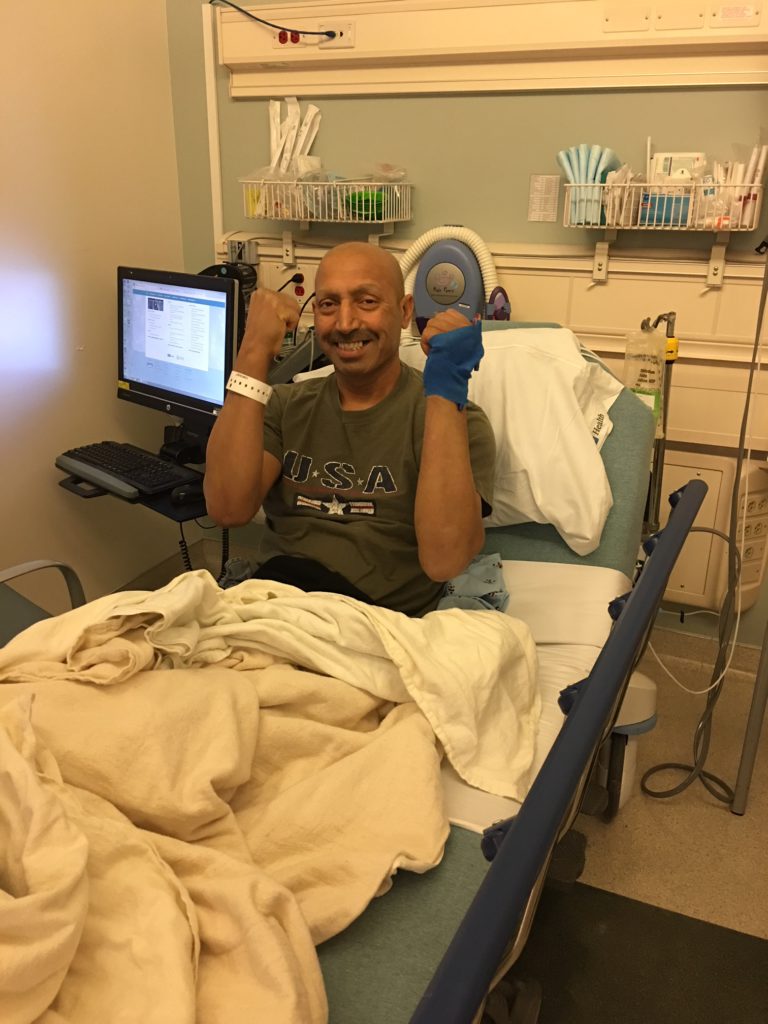
CAR T-Cell Treatment & Results
What happens during CAR T-cell therapy?
Once [the pharma company] makes the calendar, the first part of the process is called leukapheresis. It is the process in which the T-cells are extracted from the body and separated from the rest of the blood.
It is much like collection for a stem cell transplant. One tube goes from the body into an ATM-looking machine, where it gets separated and the T-cells are collected. Another tube takes the remaining parts of the blood from the machine and sends it back into the body. That process takes about 4 hours.
As soon as the T-cells are collected, staff and a [drug company] rep triple-verify all of the information. It’s very comforting to know his T-cells won’t get mixed up with someone else’s in the lab by mistake! Once that is done, the bag is placed into this ultra-modern Styrofoam cooler and sealed.
The [drug company] rep immediately takes it to the airport so it can be sent to the lab immediately. The T-cells are frozen, so it is time and temperature sensitive. The T-cells spend about 17 days in the lab, including travel time.
Shahzad went back to work for 2 weeks. On December 21, he was taken into surgery to have his 2-year-old internal port removed and replaced with an external Hickman line.
Then on December 22, he began the preconditioning chemo known as Cy/Flu for short. It is done outpatient and took a few hours each day for 3 consecutive days. Everyone responds differently. He had some fatigue and nausea and vomiting.
Receiving the T-cells
He had Christmas day free to recover. On December 26th, he was admitted to the hospital and prepped. On December 27th, that cooler was again retrieved at the airport and brought to the hospital by the [drug company] rep. Again, everything was triple-verified before removing the bag from the cooler.
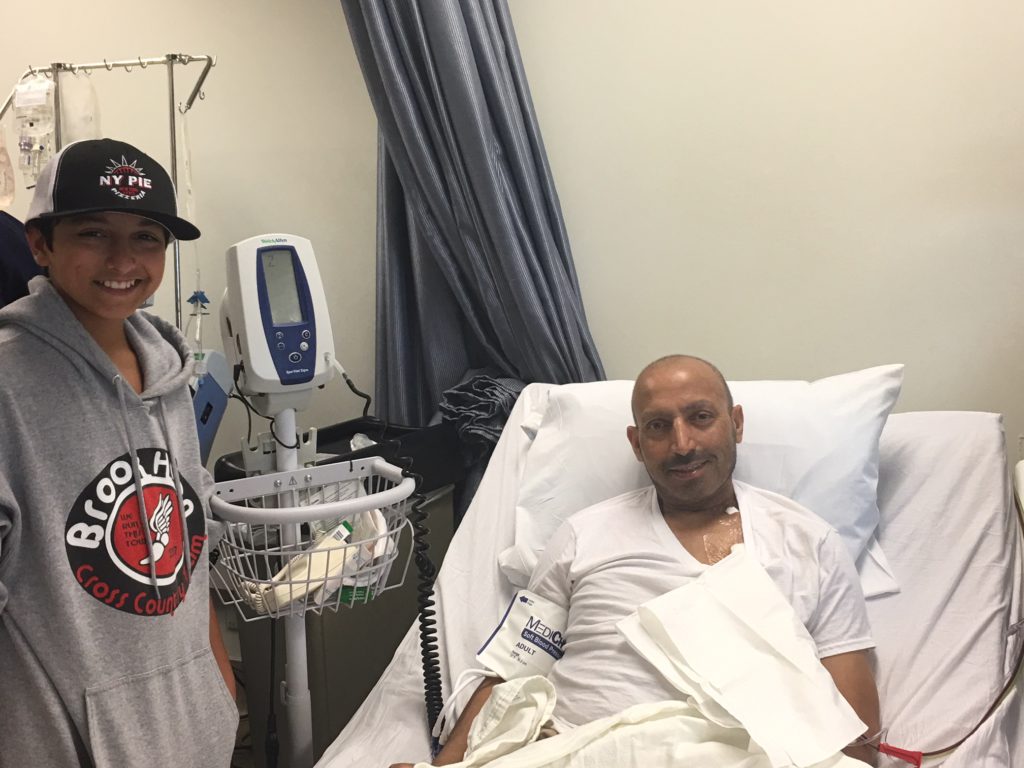
It really was quite the production! At that point, we were advised that it is common to smell an odor once the T-cells are infused. Some smell creamed corn. Some smell tomato soup.
The bag contents are clear — T-cells are microscopic — and frankly, it looked empty. I joked that this empty bag costs $373,000! The tubes were hooked up and in went the T-cells. It was actually very anti-climatic.
Was there physical pain during CAR T-cell therapy?
There was absolutely no pain involved for him whatsoever. The only pain he ever had was in the beginning when the lymphoma decided to go into his foot bone, and he couldn’t walk.
Compared to the harsh chemo he went through and what a stem cell transplant has been described as, this was the easiest piece of cake ever. He was ready to return to work in 5 weeks.
What were the side effects?
Typically, nothing happens for the first 3 days, but that’s an average and everyone is different. There are 2 major side effect potentials: cytokine Release Syndrome (CRS,) also known as “the Storm.”
This is where the T-cells are working, but the body is kind of like, “Whoa, what’s going on?” One may develop flu-like symptoms, including weakness, fever, etc.
The other is neurotoxicity, where the cytokines mess with the brain. This can cause confusion, loss of memory, seizures or cerebral edema. They can be very serious, but 20% in trial never experienced any.
The good news is it is temporary, patients are very closely monitored, and changes are addressed and treated immediately. For example, if neurotoxicity begins, the neurologist is engaged immediately.
They may administer a steroid or run a CT scan to stop the symptoms. In most cases, a patient is kept admitted for 7 days, longer if needed.
Once discharged, the patient must remain in the immediate area for 21 days for observations and daily blood counts. There are several medications prescribed.
Some start prior to infusion, some start at infusion, some end at post-30 days, some may start at post-30 days, and one is for a minimum of 6 months, Acyclovir, which is the only one Shahzad was put on.
Some take with food, some on empty stomach. You really must be very organized to manage this. There are also several “as needed,” but he never needed them. On the days that he did not visit the outpatient clinic, he did need to flush his PICC line with Heparin.
Low white blood counts and low potassium are common after infusion, and are treated. There will be a PET scan sometime around Day +30. In many cases with Yescarta, patients are seeing complete metabolic response.
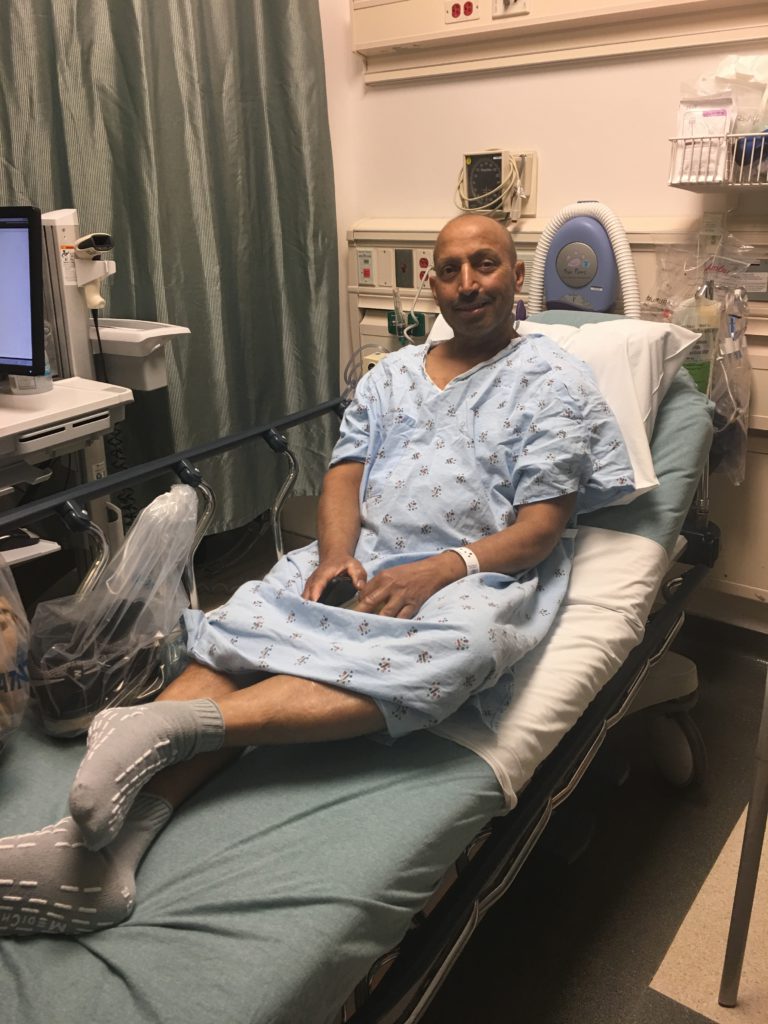
Were there any surprises?
The only part that was difficult was when he had an episode that was originally thought to be a seizure. They did everything they told me would be done: brought in a neurologist, gave tocilizumab, ran a CT scan, etc.
It turned out not to be neurotoxicity, but for the hours that we thought it was, it was petrifying. I was warned and knew to expect it, but I don’t think there is any way a human can really prepare for the emotions that come when actually seeing it happen.
What do you wish you had known before CAR T-cell therapy began?
For me, nothing other than the emotions from the episode. Everything else went exactly as planned. I wouldn’t change it and have absolutely no regrets!
Quality of Life
“Be your own advocate”
You need to be your own best advocate. You can’t rely on others to hand you information. You need to go out and look for it yourself. There are so many out there and free resources to help you.
Do your research. Learn as much as you can and question everything, because people do make mistakes, even doctors and nurses. I would definitely suggest a second opinion to be 100% sure.
Each patient is different. Each subtype is different. Each experience is so different. You can’t compare yourself to a graph or statistics — it just doesn’t add up.
Did your husband work during CAR T-cell therapy?
No, but he was ready for work 5 weeks later. Also, law prohibits a patient from driving for 60 days after infusion, so we waited until he could drive to return to work, but by then his counts were normal and healthy.
»MORE: Patients talk about working during cancer treatment
Lack of support
Dealing with friends who didn’t engage. It took me a really long time to understand why. Cancer isn’t contagious, so there was no physical risk.
They are not people who are “fair-weather friends,” so why the lack of interest? I truly believe they haven’t encountered this type of illness in their life, so it’s just too painful to acknowledge it. I believe it’s merely self-preservation, and I get it.


Inspired by Shahzad's story?
Share your story, too!
Non-Hodgkin’s Lymphoma Stories
Tony W., Relapsed T-Cell/Histiocyte-Rich Large B-Cell Lymphoma (T/HRBCL)
Symptoms: A lot of effort needed cycling, body wasn’t responding the same; leg swelling
Treatments: R-CHOP chemotherapy, CAR T-cell therapy
Symptoms: Chest pain, back pain, bump on neck, night sweats Treatments: Chemotherapy, CAR T-cell therapy




One reply on “Shahzad’s Stage 4 Refractory DLBCL Non-Hodgkin’s Lymphoma Story”
Hi, I have finished the Car T cell transplant and everything went exactly as planned. I have no sign of disease after 3 PET scans.