AML Biomarkers: How Testing Shapes Your Treatment Options
Watch the Replay On DEMAND
Listen in as AML expert Dr. Stephen Strickland from Sarah Cannon Research Institute, AML patient and doctor Joseph, and advocate Steve Buechler discuss how biomarker testing can unlock better options. Learn how understanding what mutation you have, like NPM1, IDH, KMT2A, and FLT3 can shape treatment choices—and how patients can work with their doctors to explore every option, including clinical trials.
Key Topics Covered:
- Understand AML Biomarkers – Learn how FLT3, NPM1, IDH1/2, and KMT2A mutations impact risk and treatment choices
- Test Early, Treat Smarter – See how early biomarker testing shapes decisions from day one
- Explore Targeted Treatment Options – Understand how biomarker-driven therapies are changing care
- Navigate Clinical Trials with Confidence – Learn how to evaluate opportunities and what trial participation really looks like
- Partner with Your Care Team – Get tips to advocate for testing and align on a personalized treatment path

Table of Contents
Introduction
Stephanie Chuang: There’s so much going on at and around diagnosis. There’s a lot to digest and so much information coming at us. We hope that you walk away from this discussion empowered to improve your AML care by knowing more questions to ask your healthcare providers or maybe to advocate for yourself, to understand how to be more engaged in your care, and to know your treatment options from biomarkers through clinical trials.
I’m the founder of The Patient Story. More importantly, I’m a blood cancer patient advocate myself. While I wasn’t diagnosed with AML, I was diagnosed with non-Hodgkin lymphoma and I remember swimming in questions. Things were happening so quickly and it felt so difficult to understand what was what.
We don’t know what we don’t know and that’s why The Patient Story aims to build education and community through educational discussions and hundreds of in-depth patient stories, all with the goal of amplifying the voices of patients and care partners.


For this discussion, our patient advocate Steve Buechler talks with a leading expert in AML treatment, Dr. Stephen Strickland from Sarah Cannon Research Institute (SCRI) and one of his patients, Joseph.
While we hope that this discussion is helpful for you, keep in mind that this is not meant to be a substitute for medical advice, so please consult with your healthcare team about your decisions.
Steve is going to help guide this discussion, but we all agree that it would be helpful to understand his story first. Steve, as an AML patient advocate, we’d love to hear more about what brought you to this point in sharing your story.
Not only did I not have nonspecific symptoms, but I didn’t have any symptoms whatsoever… If not for that physical, I probably would have learned about my AML when I was in much rougher shape.
Steve Buechler, AML Patient
Steve Buechler: I was diagnosed in June 2016. I did what doctors tell you to do, which is to get an annual exam. I had no symptoms whatsoever and thought I didn’t need to do it, but I kept the appointment. As part of the routine annual physical, a complete blood count (CBC) test was performed, and it was found that my white blood cell count was dangerously low. They referred me to a hematologist-oncologist who did a bone marrow biopsy, which detected the AML with 50% blasts.
From there, we were off to the races. I was admitted to the hospital the next day and was hospitalized for 37 days with the initial 7+3 treatment and eventually, a stem cell transplant. Not only did I not have nonspecific symptoms, but I didn’t have any symptoms whatsoever. Maybe I was a week away from having symptoms, who knows? If not for that physical, I probably would have learned about my AML when I was in much rougher shape.
Now, more than ever, knowing your biomarkers, mutations, and genetic alterations is absolutely essential. I heard there are dozens of different subtypes of AML, based on how those are present or absent. Many of them respond to different treatments, so you have to know that information if you want to find a targeted treatment that’s right for you.


We’ll be talking about the importance of testing for biomarkers in AML treatment and how doctors and patients can work together to determine what options are best for them. We’re joined by Dr. Stephen A. Strickland, the Director of Leukemia Research for Sarah Cannon Research Institute. He’s an internationally respected leukemia researcher with more than 100 peer-reviewed scientific publications and abstracts. Also joining us is Joseph, one of Dr. Strickland’s patients. Joseph, can you tell us a bit about your journey with AML?
Joseph A., MD: My story started when I was coaching mountain biking two summers ago. I noticed that my legs were becoming very fatigued very quickly. During a mountain bike race, I started having chest pain, which led me to the emergency room and yielded a CBC test that showed extreme concerns for a blood cancer.
Immediately, I was flown to Sarah Cannon under the care of Dr. Strickland and it took off from there. It’s a wild ride to find out you have leukemia, then to find out that you don’t have months or even weeks to think about and weigh the treatment options. Over the course of several days, I had to make some pretty aggressive decisions and start treatment. It was a whirlwind.

What are Biomarkers in AML?
Steve: We’re going to start by looking into biomarker testing. Dr. Strickland, what are biomarkers and what are the different types? We hear about diagnostic, prognostic, predictive, and monitoring. It sounds pretty complicated. Can you sort it out for us?
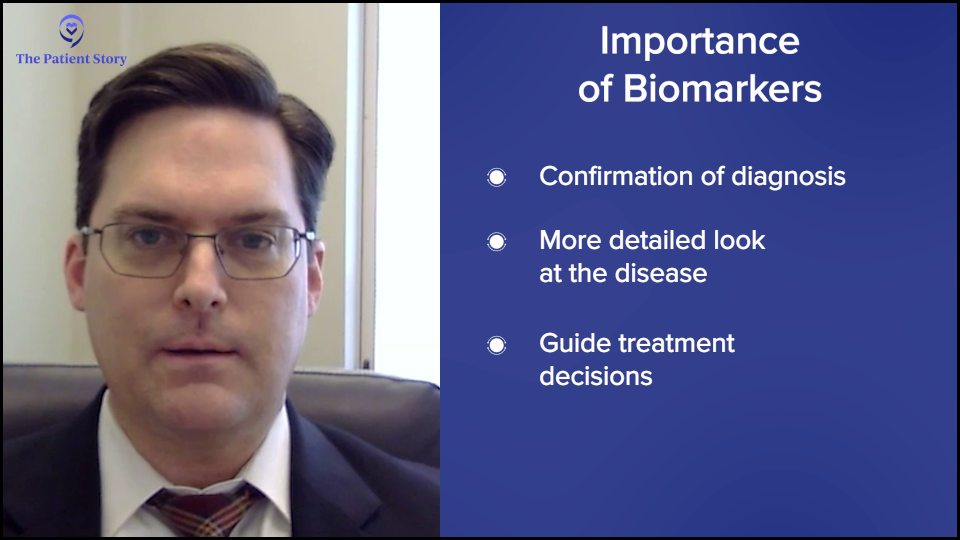
Dr. Stephen Strickland: You hit it on the head. It’s complicated. Historically, as we looked at AML, it was diagnosed simply by looking at cells under a microscope. We know that those cells can look similar under the microscope, but the biology of AML is very heterogeneous and diverse. Looking at these biomarkers can help us understand the true heterogeneity of those cells and now help us to predict prognosis as potential therapeutic opportunities.
There are different biomarkers that have been developed over the years. Now, we have a broader spectrum of testing that we do on every patient as standard of care at the time of initial diagnosis to help guide us in our treatment decision-making.
What Biomarkers are AML Patients Tested For?
Steve: For AML in particular, can you describe what type of biomarkers you commonly test for?
Dr. Strickland: There are different types of biomarkers and what we’re looking at is the biology of the leukemia cell. There are tests where we look at the cytogenetics or karyotype, which is a chromosome analysis of the leukemia cell, to see if there are specific genetic aberrations. Those genetic changes may inform us if someone is going to be a favorable risk, intermediate risk, or high risk.
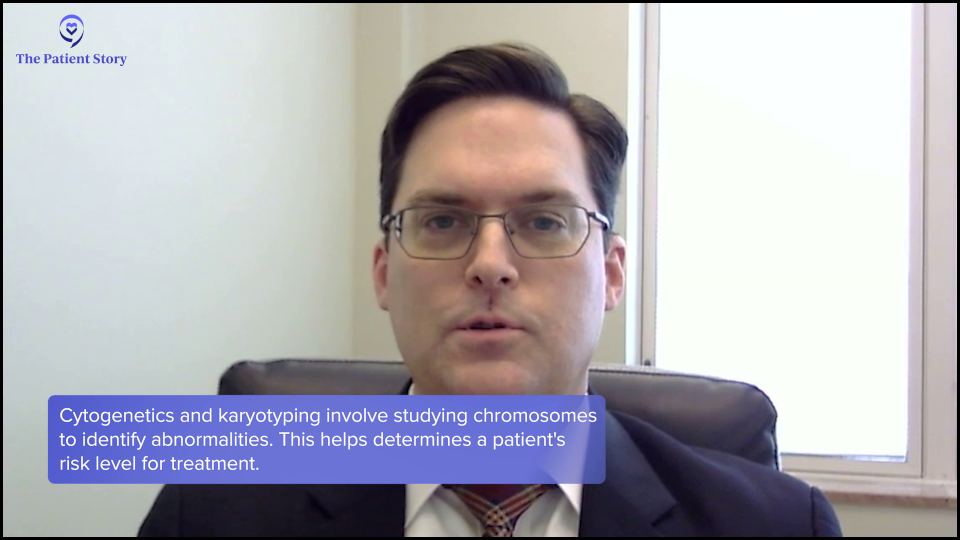
A patient’s risk classification can have implications for therapies that we may want to consider, including clinical trials that we may want to consider from the very beginning. But it also helps inform us, once we hopefully have the patient in remission, whether or not we need to consider something like a stem cell transplant to give them the best chance at a cure.
We’ve had access to cytogenetics for several decades now. More recently, molecular profiling based on PCR (polymerase chain reaction) tests, single-gene assays, and next-generation sequencing (NGS) has been developed. NGS can assess anywhere from 35 to 200 molecular mutations on these panels, giving us a vast amount of information.

Several mutations are important and, ever since the early 2000s, have been encouraged to be tested at initial diagnosis, like the FLT3 mutation. We know that patients who have FLT3-ITD can often have higher-risk disease biology, especially before the availability of FLT3 inhibitors.
IDH inhibitors are now available for patients who have IDH1 or IDH2 mutations, which has been historically and predominantly in the relapsed/refractory setting. Data is now looking at the incorporation of IDH inhibitors into frontline therapy. More recently, understanding NPM1 as a prognostic factor, but with the advent of menin inhibitors, we look at the availability of NPM1 as a potential therapeutic target.

Recently, in the relapsed/refractory setting, we had the approval of revumenib (Revuforj), which is the first targeted therapy for patients with KMT2A-rearranged relapsed/refractory acute leukemia. We’re looking at combinations of this menin inhibitor with other menin inhibitors in the frontline space, which are known in more advanced leukemia and have activity. Can we do better for patients by incorporating them in the frontline setting?

There was recent data at the 2024 Annual Society of Hematology meeting where multiple menin inhibitors were presented in a relapsed/refractory setting with single-agent activity as well as in combination therapy in a relapsed setting and a frontline setting.
Two menin inhibitors, bleximenib and ziftomenib, had data presented in combination with conventional, intensive induction therapy in the frontline setting with very high response rates and more importantly, good complete remission (CR) rates and also MRD-negative CR, which is the deepest remission we can test for, being achieved at a high rate for NPM1-positive patients.
Also, in patients who have KMT2A rearrangement, which is a specific genetic rearrangement that can often confer higher risk, we’re seeing high response rates in combination with conventional, intensive induction therapy plus menin inhibitors. This specific area has been an area of struggle for us because many of these patients were resistant to conventional chemotherapy and now, it seems like we’re finally breaking down the door and helping more patients.
Do Age and Gender Play a Role in Biomarkers and Mutations?
Steve: Do any of these mutations or biomarkers cluster into specific groups of people, by age, gender, or other social factors?
Dr. Strickland: There are a variety of factors that we can see. NPM1 can occur across the age spectrum, but the other aspect is what mutations or other abnormalities are occurring simultaneously and that has an influence. By itself, NPM1 is thought to be a favorable risk feature. But there’s also data that suggests that when NPM1 co-occurs with an IDH mutation or a DNMT3A mutation, its positive impact may take a hit based on the co-occurrence of these other mutations.
Let’s take, for example, NPM1 that co-occurs with a FLT3 mutation. FLT3 by itself puts patients into a high-risk category, but FLT3 plus NPM1 puts them in an intermediate risk, whereas NPM1 patients by themselves go into a favorable risk. It’s not a single mutation that’s driving it. You have to look at the bigger picture of the molecular profile and the diversity within a patient’s leukemia to see the impact and open the door for additional therapies.
Historically, KMT2A rearrangement has been associated with patients who have received prior anthracyclines. Some patients who receive prior anthracyclines will have KMT2A rearrangement, which confers a higher-risk disease. The availability of these therapies for a traditionally high-risk patient population is going to be very important.
You have to look at the bigger picture of the molecular profile and the diversity within a patient’s leukemia to see the impact and open the door for additional therapies.
Dr. Stephen Strickland, AML Expert Oncologist
Steve: Interesting. My treatment was in 2016 and they ran me through the traditional 7+3. They tested for FLT3, which was not present and was a good thing, and NPM1, which was also not present. That’s what landed me in the intermediate risk category and made for a more complicated decision, along with a normal karyotype. None of which I understood at the time. I’ve since come to understand it, but it’s a lot to learn and take on.

How and When Do You Test for These Biomarkers?
Steve: How and when do you test for these biomarkers?
Dr. Strickland: Testing at diagnosis is critical to help inform prognosis, but as the treatment landscape has changed over the years, it can also inform treatment decisions from day one. It’s strongly encouraged and I’d say a necessity to do at the initial diagnosis. Even if we don’t get the information back, like when we have to start treatment in an emergent situation, that information can still be obtained. If the patient responded to the initial therapy and achieved remission, if we don’t have their biomarker information, we can’t always inform prognosis and/or recommendations, like whether or not they need to proceed with a stem cell transplant.
We check at the time of presentation to confirm the initial diagnosis and help us understand the characteristics of the disease. But for some of these tests, we’ll reassess, even when the patient is in remission, to help us understand the depth of remission. Are patients achieving a very deep remission, which is the goal of our therapy?
We can do some of this testing on peripheral blood, especially for interval follow-up to minimize the number of bone marrow biopsy procedures patients have to undergo.
Dr. Stephen Strickland, AML Expert Oncologist
Steve: Is this typically done through a bone marrow biopsy or some other technique?
Dr. Strickland: Typically, we would do it at the time of the bone marrow biopsy on the liquid portion that we achieved during that process, but it can also be done on peripheral blood. There are ongoing debates about one being more sensitive than the other, but bone marrow biopsies are not always the most fun to undergo repeatedly, though sometimes a necessity. We can do some of this testing on peripheral blood, especially for interval follow-up to minimize the number of bone marrow biopsy procedures patients have to undergo.
Because of my previous exposure to chemotherapy, we elected to go with a more aggressive chemotherapy regimen right out of the gate… the genetic markers guided his recommendations.
Joseph A., AML Patient
How I Processed My AML Diagnosis
Steve: Joseph, how long after your diagnosis before you were tested? Through that process, what were your thoughts? Did you have any fears or anxieties? What was it like to live through that phase?
Joseph: Mine was a little more complicated than average. I had a childhood malignancy for which I received chemotherapy. I fell into an atypical category in that mine could have been and may still be treatment-associated leukemia, which could fall into the higher-risk category.
I was admitted in the middle of the night after being helicoptered to Sarah Cannon. On day one, Dr. Strickland stepped into the room and said, “We’re going to look for markers. This is what I’m thinking. There are some newer drugs for these different markers.” We sat down and talked about those markers within a day or two of the bone marrow biopsy.
Because of my previous exposure to chemotherapy, we elected to go with a more aggressive chemotherapy regimen right out of the gate. My case is a little bit skewed, but the genetic markers guided his recommendations of newer medicines versus the overall course of my therapy and even the choice that we ultimately made about stem cell treatment.
Barriers to Biomarker Testing
Steve: Dr. Strickland, despite its importance, early biomarker testing is not universally standard. What kinds of barriers or limitations exist to implementing this as a routine practice and how can they be addressed?
Dr. Strickland: It’s gotten better over the past several years as we’ve tried to get the word out. It’s listed in the NCCN Guidelines to be able to get a baseline assessment on patients at the time of initial diagnosis. But it also has some implications as far as where a patient is presenting, where the initial bone marrow biopsy was done, and whether or not the hospital system has access to those tests.
It’s not uncommon to repeat the bone marrow assessment to ensure that we get the testing done. But all of the providers who are coming in contact with these patients advocate with the hospital system and the pathology laboratories to ensure that we get this testing done. It’s critically important in the decision-making process.

AML Standard of Care and New Treatments
Steve: We’re going to shift gears to treatment options for newly diagnosed and relapsed/refractory AML. Dr. Strickland, once a patient has undergone testing, what are their treatment options? Before we get into specifics, can you give a broad overview of what has been the standard of care and what we might be shifting to, including targeted therapies or other options?
Dr. Strickland: The conventional induction therapy that we use is a regimen called 7+3. It’s easy to remember because some of the first publications about it were in 1973. It’s seven days of backbone therapy of cytarabine and three days of an anthracycline, either daunorubicin or idarubicin.
[In the past] If patients were not fit enough to receive therapy, they were often not offered any chemotherapy and sometimes went directly to hospice.
Dr. Stephen Strickland, AML Expert Oncologist
For many years, that was the treatment of choice and it was a one-size-fits-all therapy. If patients were not fit enough to receive therapy, they were often not offered any chemotherapy and sometimes went directly to hospice. With a diagnosis that has a median age in the upper 60s, you can imagine that quite a few patients were sometimes not offered therapy if they weren’t fit for intensive induction.
But in 2017, with the approval of midostaurin (Rydapt), that started to change, at least for patients with FLT3 abnormalities that were identified at the time of their initial diagnosis. The approval of that drug added a targeted therapy, an FLT3 inhibitor. As a result, that began changing the landscape.
Now, that’s a subset of patients — about 25 or so percent of AML patients fall into that category. We’ve continued to work towards identifying other markers and developing new targeted therapies.
The landscape has changed with the approval and availability of medicines like gemtuzumab (Mylotarg) and other medicines that have come on the market to help guide us for favorable-risk patients who may get an additional antibody therapy. Gemtuzumab, in addition to conventional therapy, is for FLT3 patients who may get an FLT3 inhibitor. We also have midostaurin and quizartinib (Vanflyta), which are now approved in the frontline setting.
We’ve also had therapy advances for patients who are not fit for intensive therapy with the availability of hypomethylating agents (HMAs), like azacitidine (Vidaza, Onureg), decitabine (Dacogen), and even low-dose cytarabine (Cytosar) in combination with venetoclax (Venclexta), a BCL2 inhibitor.
Based on a patient’s fitness level and molecular or cytogenetic profile that the leukemia may possess, we have multiple agents to help us hone in and take advantage of those markers that are present to guide the patient to the most effective therapy.
Promising Areas of AML Research
Steve: What are some of the most exciting spaces that are either newer options that are already approved or those that are promising in research in phase 2b or 3 trials? Does it make a difference? Can you distinguish what’s for newly diagnosed patients as opposed to relapsed and refractory patients?
Dr. Strickland: There’s a lot of work that’s being done in this space and across different spectrums with different mechanisms of action. There are areas of opportunity in terms of how we can harness the power of the immune system with either antibodies or antibody-drug conjugates (ADCs).
We’re also developing cellular therapy options, whether it’s the patient’s immune system being educated to identify and attack the leukemia cells or whether a healthy donor donates immune cells that can be engineered to hopefully attack a certain characteristic of the leukemia cell. We’re seeing this across different tumor types and across the landscape of oncology in general.

But one very exciting area that we’re dealing with is a new small molecule inhibitor called menin inhibitors. This is being looked at in the relapsed/refractory patient population and is showing activity as a single agent, being able to achieve deep remissions for some relapsed/refractory patients. Relapsed/refractory patients are those who have undergone prior conventional therapy and whose disease has persisted and/or relapsed despite the conventional therapy.
This opens up an opportunity. If menin inhibitors benefit relapsed/refractory patients, can we add these to conventional and frontline therapy? Can we get these medications to patients earlier on to hopefully help them achieve remission more commonly and achieve a deeper remission to hopefully impact overall survival?
Menin inhibitors are very popular nowadays in the world of leukemia. Every conference has a session with multiple companies that are developing menin inhibitors because it’s a very exciting space.
We’re trying to take advantage of the biological characteristics of a patient’s cancer, so there are a lot of studies that are biomarker-driven.
Dr. Stephen Strickland
The Role of Biomarkers in Clinical Trials
Steve: How do biomarkers play a role in clinical trials? Can they make you either eligible or ineligible for certain trials? How do those two things work together?
Dr. Strickland: It’s definitely been an evolution in the leukemia research space, the clinical trial space, and oncology research in general. We’re trying to take advantage of the biological characteristics of a patient’s cancer, so there are a lot of studies that are biomarker-driven.
If a patient’s malignancy has a specific marker, then we try to get them access to targeted therapies. Sometimes these drugs may be more effective on a broader scale. With the initial development, we’re also trying to enrich the population of patients who are truly going to, hopefully, benefit from the medications.
We’re trying to take advantage of the characteristics we now can better understand about one person’s leukemia that we didn’t know existed, say, 30 to 40 years ago. Now, we have the technology to help us identify these biomarkers and identify them relatively quickly, which can be instrumental in guiding us to either commercially available therapies or clinical trial participation where there may not be something that’s already FDA-approved.
How Biomarkers Guide Treatment Decisions
Steve: How does knowing someone’s biomarkers impact their treatment choices? Do biomarkers give you some idea about the potential effectiveness or potential side effects of treatments?
Dr. Strickland: Biomarkers can definitely help to guide the therapy decisions, not so much from a side effect profile but more so from an efficacy perspective. The way that I view traditional therapies is like going to battle with a tank. Tanks can be very effective, but they’re not very specific. Some of these newer therapies, these targeted therapies like FLT3 inhibitors, menin inhibitors, and IDH inhibitors, are like adding a sniper to the mix. Hopefully, by going to battle with both tanks and snipers, we can have a more effective outcome against this malignancy.
As the final testing came back, I found out that I might be a candidate for one of the new menin inhibitors, which was very exciting.
Joseph A., AML Patient
Working with a Doctor on Treatment Decisions
Steve: Joseph, how did you work with Dr. Strickland to decide on the best treatment options and how did it influence treatment choices?
Joseph: Right out of the gate, we knew that I was going to start in the high-risk category, so we started with the typical chemotherapy regimen. But as the results of my biomarker testing started to come back, every day during rounds, I would anticipate another conversation with Dr. Strickland and his team.
As the final testing came back, I found out that I might be a candidate for one of the new menin inhibitors, which was very exciting. I could start with a tank and a sniper right from the beginning. That’s the route that we elected to go.
When you’re starting to learn what the next three to six months of your life would look like, then you can settle in and realize the path that’s in front of you.
Joseph A., AML Patient
Addressing Fears and Concerns During Treatment
Steve: That does sound promising, but nonetheless, what were some of your biggest concerns during the process? It had to be an anxiety-provoking time.
Joseph: Anxiety doesn’t even cover it. Steve, you might know yourself. You go from somebody healthy, active, and surrounded by people you love, then you’re put in this unfamiliar environment, told you can’t leave the floor because you’re going to be neutropenic in several days, and can’t have contact with the world or your typical support network.
Every day, as you’re discovering a little more about your disease, there are very few familiar faces around you or familiar environments. It was a very anxious time. But at the same time, I found hope as we talked about some of these findings. I could have found out that I had multiple mutations and was a candidate for none of the new drugs and that we were going to try chemotherapy. If that didn’t work, here was the backup plan and here was the backup for the backup.
There was no end to the initial anxiety, if you will. About three to four weeks in, when you’re starting to learn what the next three to six months of your life would look like, then you can settle in and realize the path that’s in front of you.

Addressing Common Concerns from AML Patients
Steve: Dr. Strickland, on your side of this collaboration, what are some of the most common questions that you get from patients going through this process?
Dr. Strickland: It’s such an overwhelming time for many patients. Acute leukemia, in general, is cruel in the sense that patients don’t go into the hospital or the doctor’s clinic expecting to get that diagnosis. They walk in with nonspecific symptoms and often think there’s something else.
Then they’re told they’re going to be sent to a leukemia center and they’re going to undergo a biopsy. Before they even have time to digest the word leukemia or cancer in general, they’re being told they’re starting treatment in the next day or two, and we start talking about all of the side effects before many patients and their families have time to process the information.
We’re trying to navigate and walk hand-in-hand with the patient and their families as we get this information.
Dr. Stephen Strickland, AML Expert Oncologist
With other malignancies, patients and their families have weeks to digest the information and come to terms with it. But in the world of acute leukemia, we often have days to maybe a week, if we’re lucky, but often it’s less than a week when all of this happens. Then we talk to patients and their family members about a clinical trial or different therapies and what we have to offer. It’s a very overwhelming time.
We’re trying to navigate and walk hand-in-hand with the patient and their families as we get this information. What are we looking for? What is it going to mean? How are we going to use this information? In Joseph’s case, we understood that there was prior chemotherapy exposure, so there’s a possibility that this is a therapy-related leukemia, which guided our choice of induction therapy. Eventually, some of the biomarker testing came back and, as a result, we were able to access a particular trial with a menin inhibitor that allowed patients to start conventional backbone therapy.
If we had the information about the biomarker, then we could enroll the patient and get them access to this other targeted therapy, so to speak. It’s a huge effort to try and coordinate this. There’s communication and coordination even with the sponsors of the trial to set it up in a way that we have a little bit of flexibility on the front end. We didn’t burn a bridge for someone who we wanted to and felt the need for starting therapy quickly but yet still could have access to some of the more cutting-edge therapies that are coming up as well.

How Care Partners Influence Treatment Decisions
Steve: We’ve talked about providers and patients. Let’s bring in a third group here because at The Patient Story, we’ve often talked about getting loved ones involved in the process. Joseph, can you say a bit about who your caregivers or care partners are and how they help influence or inform your decisions?
Joseph: My wife is an office manager for a medical group. It was beautiful because they gathered together and said she could stay with me and manage the office remotely. She stayed by my side the whole time, which was phenomenal and honestly helped me make a lot of the tough decisions.
There was nothing left off the table. Here are the good and the bad. Here are all the options.
Joseph A., AML Patient
In addition to that, I’m a physician as well, so I had access to a lot of different doctors whom I could call and ask about certain things. I called a buddy of mine who’s a vascular surgeon when I was picking out whether I wanted to port-a-cath or a temporary line in my arm. I pulled some strings that way to make my decisions.
But ultimately, I felt so comfortable with Dr. Strickland. When he entered the room the very first time, he laid it all out. I felt like there was nothing left off the table. Here are the good and the bad. Here are all the options. Then, as more information came back, I felt like he immediately updated me on what the new information was and what the options were. My wife Heather and I felt like we had all the information we needed to make the best decisions for my care.
Since I had no basis for choosing one over the other, I agreed to join the trial.
Steve Buechler, AML Patient
How Can Patients Learn About and Navigate Clinical Trial Opportunities?
Steve: Let’s talk about clinical trials. I was in a clinical trial myself. We reached a point where I decided I wanted a transplant, but we had to select a donor. I had a half-matched sibling, my brother, and they said he’s a potential donor, but we should also consider umbilical cord blood donors. It sounded like science fiction to me, but they convinced me it was a plausible alternative. I asked which one’s better and they didn’t know, but there was a clinical trial to figure that out and I could join the trial.
Since I had no basis for choosing one over the other, I agreed to join the trial. They randomly assigned me to the cord blood option, so my brother, as a donor, was off the hook. My fate was in the hands of a mother and baby who donated their umbilical cord blood. All ended well, but ironically, years later, the results of that trial came out and they slightly favored half-matched siblings as opposed to umbilical cord. I was an n-of-1 and the umbilical cord blood worked for me.
Clinical trials are crucial to figuring out what works and who benefits from what works. Dr. Strickland, when and why are clinical trials a good option for a patient and how can patients find and evaluate trial opportunities?

Dr. Strickland: I’m going to be biased, but I will say that when available, clinical trials are going to be appropriate for any patient with acute leukemia and every patient with acute leukemia. We’ve had drugs that have been around since the early 70s or even earlier that we’ve utilized, but while some of these agents can be effective in getting patients in remission, we know that for many patients, a cure can be elusive.
Unfortunately, the majority of patients with acute leukemia end up relapsing, but that’s also because many of them fall into an intermediate risk or higher risk group. When that happens, we know we can get them in remission, but the question is: how do we keep them there? That’s where a lot of the effort is to try and modify frontline therapy to achieve deeper and hopefully more sustainable remissions.
If they have well-tolerated medications, maybe those medicines can be used as maintenance therapy. Those will allow the patients to stay on a low-intensity, well-tolerated therapy for a longer period and hopefully exhaust that leukemic clone altogether.
As we get the information about the biology of any patient’s leukemia, if we have a trial option, we want to try to swing the pendulum as much in their favor and give them a benefit.
Dr. Stephen Strickland, AML Expert Oncologist
I’m very passionate about the idea of clinical trials. I’m going to be biased because I’m a clinical trialist at heart. We want to offer the best available therapies to our patients. As we get the information about the biology of any patient’s leukemia, if we have a trial option, we want to try to swing the pendulum as much in their favor and give them a benefit.
But there are patients with leukemia who are treated throughout the country and the world without access to clinical trials. Sometimes, we also deal with patients in a relapsed/refractory setting. In that area, it’s even more crucial to try and gain access to clinical trials.
Historically, many of our relapsed/refractory therapies have about a 30% chance of getting the patient in remission, which means the majority of patients in a relapsed/refractory setting will not achieve remission with the salvage therapy. Better therapies, more effective therapies, and/or combinations of therapies to take advantage of the biology of the disease are crucial to be able to offer to patients.

How Can Patients Learn What Clinical Trials are Available?
Steve: Dr. Strickland, it sounds like your patients are going to hear about clinical trials, but for other patients who may not have a doctor who’s a clinical trialist, how should they go about finding and evaluating clinical trial opportunities? What’s a good source of information?
Dr. Strickland: There are plenty of resources out there, but with the Internet, you have to be cautious about what you Google. The Leukemia & Lymphoma Society is instrumental in trying to help provide patients with education about opportunities, and they obviously support quite a bit of research as well.
ClinicalTrials.gov is a website where all clinical trials in this country are registered. With its search engine, you can input a few keywords and your diagnosis to get information about trial opportunities available. There’s also additional information about the sites that are participating in each of the different clinical trials.
We’re here trying to offer them access to the most cutting-edge therapy that they can have access to and that’s where my passion is.
Dr. Stephen Strickland, AML Expert Oncologist
The information is out there if you know where to look, but the biggest thing is talking with your oncologist. Patients need to be informed and empowered to ask questions. Where is the nearest leukemia center near me? What are my trial options?
We’re trying to get away from people thinking that they’re guinea pigs when they go into a study. We’re not trying to experiment on patients. We’re here trying to offer them access to the most cutting-edge therapy that they can have access to and that’s where my passion is.
When we look at hundreds or thousands of patients who go on a study, we will be able to make informed decisions about patient populations. But when we’re meeting one-on-one with a patient, it’s about what we can do to help that individual patient and that’s where the offering for a clinical trial comes from.

What Role Does Age Play in Clinical Trial Eligibility?
Steve: Given that the typical AML patient is in their late 60s, does age impact the decision to encourage them for a clinical trial?
Dr. Strickland: It does. Age impacts it in several ways. We know that age is not the best determination of someone’s performance status or fitness, but it does play a role as far as the wear and tear of other organ systems and how well a patient can endure the side effects associated with an intensive therapy approach.
We also know that patients who are in their mid-60s and above tend to have higher-risk disease. Even if we have the opportunity to give them intensive therapy, the majority of those patients are not going to be cured with that therapy, so they’re going to be faced with potential relapse.
It does play a role as far as the wear and tear of other organ systems.
Dr. Stephen Strickland
If they’re a transplant candidate, we try to get them to a transplant. But again, as we get on the more mature range of the age spectrum, it makes the availability of something like a stem cell transplant and the intensity that goes along with it very challenging.
We need more effective therapies that can be used, for instance, as maintenance therapy. We do have that nowadays with patients who can receive hypomethylating agents plus venetoclax, which is approved for unfit patients in the frontline setting. But what do we do for patients when they progress after that therapy stops working? That’s another opportunity for clinical trial enrollment as well.

Patient Perspective on Clinical Trials
Steve: Joseph, you’re on the young end of the spectrum for AML, but how did clinical trials come up in your treatment? What did you know about them? If anything, how did you get familiar with them? How’d that go?
Joseph: Starting day one, the discussion about clinical trials was opened and what some of the possibilities were before we even knew my mutations. We immediately started talking about the opportunity of clinical trials. We talked about the whirlwind of the diagnoses, learning it, and trying to navigate and come to terms with it. I was in such shock that I was happy with any opportunity.
Unlike a traditional cancer course, there was such urgency in my case that to hear I had options, both in the traditional sense and a clinical trial, gave me comfort. They were going to be used simultaneously. We’re going to use traditional induction therapy, which we know has some efficacy, but in addition, I’m going to be considered for clinical trials and these are going to be parallel treatments. In no way did I feel like I was being experimented on. I felt very comfortable.
There are some impacts on your quality of life from being in a clinical trial… Overall, I’ve had a phenomenal experience.
Joseph A., AML Patient
As the trial went on, I loved it. There are some impacts on your quality of life from being in a clinical trial. There are checkboxes, if you will. I probably get to see Dr. Strickland’s clinic staff a little more often than the average AML patient who’s in remission. I have a bone marrow biopsy every three months, which is not ideal, but a small price to pay.
As far as side effects, I’m surprised that I’ve had minimal side effects with this drug. There was a little bit of variation in my clinical trial, almost as if they were trying to figure out the highest effective dose. There was a dose escalation partway through my trial where they tried a higher dose, but I didn’t tolerate it very well. Dr. Strickland’s team was very amenable, along with the company that runs the trial, in saying that I’ll go back down to the dose I was tolerating. That was the biggest bump in the road when they doubled and even tried to triple the dose to see if it could be tolerable and it wasn’t for me. Overall, I’ve had a phenomenal experience with this trial that I’m in.

Clinical Trials Happening Right Now
Steve: Dr. Strickland, can you describe the promising trials that are happening right now?
Dr. Strickland: We could spend a lot of time at different sites across the country with all kinds of different trial opportunities. I’ve been fortunate to be a part of several trials and also see colleagues present data on theirs as well.
The SAVE trial is looking at a triplet oral therapy for patients. It’s an oral decitabine plus venetoclax plus a menin inhibitor, revumenib, in a relapsed/refractory population that is taking away the need for daily trips back and forth to a clinic to receive chemotherapy. As some of these agents have oral formulations, being able to use a strictly oral formulation in combination therapy to hopefully impact patients and has shown very nice response rates, is opening the door for how we cater to patients as they live with this disease over time. We know that historically, they spend a lot of time in the hospital, away from work, and living their lives.

Other trials incorporate menin inhibitors into frontline chemotherapy, like the KOMET-007 trial. There’s a 7+3 plus ziftomenib that we have been fortunate enough to participate in. For patients who are NPM1 positive, the data that was presented in December 2024 suggested that 100% of patients who had NPM1-positive disease were achieving true CR and about 76% of those patients were achieving MRD-negative responses, which is very encouraging.
Now, it’s a small number of patients. We still have a lot of work to be done and we’ll see if that 100% holds up or not. Nevertheless, the fact that the majority of patients are seemingly benefiting from the therapy is very encouraging.

We also have access to relapsed/refractory studies and I briefly mentioned cellular therapy studies, like the SENTI-202 trial. We’ve been very fortunate to participate in some of these and provide access in Nashville and across our Sarah Cannon network. These therapies engineer immune cells from healthy donors to give to patients and they attack a certain characteristic commonly found on AML cells. We recently presented data that four of the seven patients who have ever received this cellular therapy product achieved deep MRD-negative remission. That’s very encouraging for patients who have had relapsed/refractory disease.
Again, a lot of this is still in its early stages, but it’s a testament to what we’re trying to do. Access to clinical trials can sometimes be better than what we have on the proverbial shelf and offer patients opportunities that they otherwise wouldn’t have.
Patient Perspective on Entering a Clinical Trial
Steve: Joseph, can you say something a bit more specific that might provide some guidance to patients who are trying to figure out whether they should think about a clinical trial? How do they decide about a clinical trial? How do they fight their way through the consent form? What’s it like from the patient’s perspective to go through the whole process of deciding and entering a trial?
Joseph: I had a good experience. Multiple staff members came to me and did a phenomenal job explaining the trial. They sent a representative to answer any questions about the potential side effects of the traditional treatment and the immunotherapy they were testing, so I thought that was great. Logistically, I found it easy to navigate the forms because there’s an expert sitting right beside you, explaining what everything means.
They want to see how efficacious it can be if they move it up in the treatment course, so I found a lot of comfort in that.
Joseph A., AML Patient
What gave me the best feeling on my trial is that I was on one of the menin inhibitors. NPM1-positive was my genetic makeup. When they presented the trial to me, they said that there isn’t half who’s getting the medicine and half who isn’t. The way it was presented was that there’s a medicine that they know works in refractory patients. The question in the trial is if it’s given at the very beginning, do you go into remission quicker and get a deeper remission?
Before I came into all of this, clinical trials were a black box. You don’t know what’s going to happen in them. The word experimentation is a daunting word. Am I going into something where they don’t even know what it does and what the side effects are? Am I going to get the real pill or the fake pill? It wasn’t like that. They know that what they’re giving me is efficacious. They want to see how efficacious it can be if they move it up in the treatment course, so I found a lot of comfort in that.
Best Advice for Newly Diagnosed AML Patients
Steve: Let’s focus on some key takeaways. I’m interested in your final thoughts. If there was one thing you wanted someone newly diagnosed with AML to know about testing and their treatment options, what would that be?
Joseph: The best advice that I could give to a patient who’s just been diagnosed, which is the advice that I followed myself and has served me very well, is to access great resources. I see that even in my practice as a neurologist. People come in after they’ve searched for their disease online with all this preconceived bad information, which is very scary.
There are great websites and professionals who can give you good information. When I headed into this dark time with a lot of confusion, I made a big promise to myself and my wife that we weren’t going to go online and search all these poor resources. We were going to trust in Dr. Strickland, his team, and his protocols. If he offered us different websites that we could look at, we would restrict our vision and our knowledge to those. I feel like you can get more solid ground under your feet by starting there, versus the huge cloud of misinformation that is the global Internet.
Access great resources… There are great websites and professionals who can give you good information.
Joseph A., AML Patient
Dr. Strickland: I would let people know that there are options. Too many times, when people hear the word leukemia, which I hear all the time, they come in very distraught. From what they know, there’s no cure. There are a lot of unfortunate predispositions or misnomers as far as what we can do. It may be because they had a family member from years ago who was told that they didn’t have any treatment options. But who knows what the specifics were?
We have many more opportunities available to us today. Approach this with an open mind. Try to understand what options are available. Hopefully, with this discussion and resources from websites like The Leukemia & Lymphoma Society and The Patient Story, you can put a wonderful face and story to this disease in the sense of what we can do and the success that we can have if we have the opportunity.
We want to empower patients. We want them to be informed. We try to inform them with our discussions early on.
I do think participation in clinical trials and getting the best available therapies as possible is going to open the door for more patients to do that.
Dr. Stephen Strickland, AML Expert Oncologist
Joseph’s story, to me, is incredible. He was living his life without any idea of what was going on underneath the surface until some symptoms developed and then the whirlwind of being transferred. One time when I called him to ask a few questions, he was very out of breath. At first, I was very concerned about what was going on. I asked, “Are you okay? What’s going on?” He said, “I’m at mountain bike practice teaching middle schoolers.”
The impact of the therapies we have nowadays and the access to a clinical trial to take someone who had to stop racing because of symptoms to the point of getting him back to doing what he loves was very gratifying. That’s the opportunity we want for all patients to have. We want them to get back to their lives and live their lives to the fullest. I do think participation in clinical trials and getting the best available therapies as possible is going to open the door for more patients to do that.

Conclusion
Steve: Thank you to Dr. Strickland and Joseph for sharing their insights. We hope this discussion has provided you with valuable information. We encourage you to continue following the latest advancements in the field and to discuss your options with your healthcare providers.
Stephanie: Thank you so much to Steve, Dr. Strickland, and Joseph for spending your time with us on this discussion that features so many perspectives. Hopefully, this was resonant with you.
We want to remind you that this program is not a substitute for medical advice and that The Patient Story retains full editorial control.
We hope this was helpful for you and that you walk away feeling more empowered to ask questions. We hope to continue having more of these conversations and to see you at another program soon. Thank you so much and take good care.
Acute Myeloid Leukemia Patient Stories
Russ D., Acute Myelomonocytic Leukemia (AMML), with NPM1 Mutation
Symptoms:Flu‑like symptoms, profound fatigue, blood pressure drop, shortness of breath
Treatments:Chemotherapy, clinical trial (menin inhibitor)
Shelley G., Acute Myeloid Leukemia (AML) with NPM1 Mutation
Symptoms: Fatigue, rapid heartbeat, shortness of breath, low blood counts
Treatments: Chemotherapy, clinical trial, stem cell transplant
Joseph A., Acute Myeloid Leukemia (AML)
Symptoms: Suspicious leg fatigue while cycling, chest pains due to blood clot in lung
Treatments: Chemotherapy, clinical trial (targeted therapy, menin inhibitor), stem cell transplant
Mackenzie P., Acute Myeloid Leukemia (AML)
Symptoms: Shortness of breath, passing out, getting sick easily, bleeding and bruising quickly
Treatments: Chemotherapy (induction and maintenance chemotherapy), stem cell transplant, clinical trials