Multiple Myeloma: Creating Personalized Treatment Options Through Clinical Trials
On Demand Replay Now Available.
- Select an “On Demand” session to watch right now.
- Select a “Replay” session to have a link sent to you.
In this joint program with Blood Cancer United (formerly The Leukemia & Lymphoma Society), join an expert-led discussion with Dr. Ajai Chari (UC San Francisco) and myeloma patient advocate Bryon Daily (Blood Cancer United) as they discuss how clinical trials are changing the standard care options for relapsed and high-risk patients.
Key Topics:
Learn the differences between 3-drug vs. 4-drug regimens and when each is recommended
Compare the pros and cons of CAR T-cell therapy vs. bispecific antibodies
Hear real patient insights from Bryon Daily, myeloma patient advocate
Get expert tips on navigating disparities in access to treatment
Ask smarter questions at your next appointment by understanding your options
By registering, you agree to let us share your contact information with The Leukemia & Lymphoma Society.

We would like to thank Blood Cancer United (formerly The Leukemia & Lymphoma Society) for their partnership.
Visit their Multiple Myeloma Overview for information and a list of resources to help you navigate myeloma.
They offer free resources like their Information Specialists, who are one free call away for support in different areas of blood cancer.
Table of Contents
Introduction
Stephanie Chuang: I’m the founder of The Patient Story. More importantly, as someone who’s dealt with her own blood cancer diagnosis, I have spent a lot of time talking with patients, care partners, and doctors about how we can make the best treatment decisions, including figuring out when clinical trials enter the conversation, especially in multiple myeloma where so much development has happened. I know firsthand how confusing and overwhelming it can be trying to navigate those options. We’re here to try and give you the information and, hopefully, the confidence that you need to have those important conversations with your care team.
Before we dive in, I want to give a big shout-out to our friends at Blood Cancer United (formerly The Leukemia & Lymphoma Society). As you probably know, they offer so many fantastic resources, including the free one-on-one support in navigating clinical trials through their Clinical Trial Support Center. I have the pleasure of speaking with many of their nurses who are there for you on the other end of that line and they are fantastic. It’s clear that not only do they know about clinical trials and how to navigate them, but they also care about these relationships and the people they connect with.
For this discussion, we teamed up with Blood Cancer United, which supported and sponsored this program, and also one of its community outreach managers, to bring you a special conversation. You’re going to hear from Bryon Daily, who was diagnosed with multiple myeloma in 2018. He had a basketball injury that then led to the diagnosis. After years of navigating treatment, Bryon has been dedicating his time to helping other people find their way after diagnosis.

He’s going to sit down with Dr. Ajai Chari, one of the leading experts in multiple myeloma. Dr. Chari is from the University of California San Francisco (UCSF), which is where I got my care in lymphoma. They’re going to talk about the latest in treatments, research happening through clinical trials, and why knowing your clinical trial options matters so much for us as patients and care partners. It’s not a last resort. It can bring tomorrow’s treatment to you today and it’s about figuring out if this is the right trial for you.
While we hope that you find this discussion to be helpful, it is not a substitute for medical advice, so please talk with your healthcare team about what’s right for you.
Bryon Daily: Our discussion will be on treatment options for patients with multiple myeloma and the importance of clinical trials in battling this disease. I’m the National Community Outreach Manager for Blood Cancer United’s Myeloma Link initiative. I’m also a six-year survivor of multiple myeloma, having been diagnosed back in 2018.
I have no history of cancer in my family that I’m aware of, so, needless to say, it was a surprise when I was diagnosed. The diagnosis was a result of my primary care physician being very diligent in following certain trends in my labs. I had escalating protein over the course of several months, but it wasn’t until about the sixth month when, while I was playing basketball at the gym, I caught the ball wrong, which hit my finger. I went home thinking it was a simple sprain.
Two weeks later, it didn’t heal; it was still as if it happened the day before, so I went to my doctor. We did imaging and it turned out to be a fracture. The fracture, paired with the escalating levels of protein, was a red flag for my primary care physician. Fortunately, he was able to refer me to several specialists.
About a month later, we ultimately went to see an oncologist who ordered a biopsy and I turned out to have myeloma. I went through several months of chemotherapy and, ultimately, had an autologous bone marrow transplant, which was successful. After several months of being out of the public domain, I began to recover.
I’m joined by Dr. Ajai Chari, a hematologist-oncologist at the University of California San Francisco. He is the Director of the Multiple Myeloma Program and Professor of Clinical Medicine. His research interests include the development of novel chemotherapy regimens, including phase 1 and 2 studies.

Look Ahead: Top Takeaway for Today
Bryon: Dr. Chari, our audience includes patients, caregivers, and partners. What is the number one takeaway you hope that they walk away with before we dive into details?
Dr. Ajai Chari: Thank you, Bryon, for having me. It’s a pleasure to be with you and thanks for sharing your story. The number one thing that patients are looking for is hope. They want to know that they’re going to be able to live as long as they want to live and with a good quality of life.
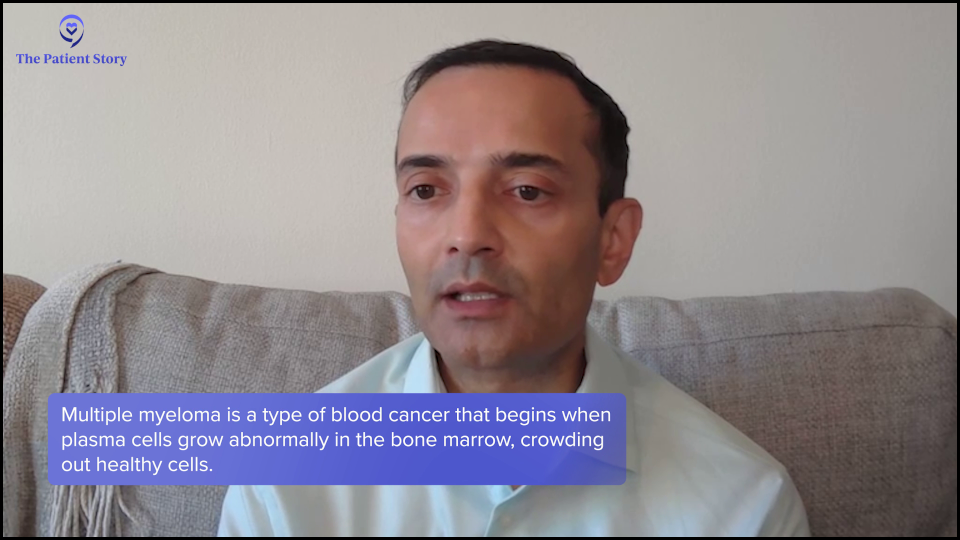
What is Multiple Myeloma?
Bryon: Doctor, what is multiple myeloma and how do we treat it?
Dr. Chari: Myeloma is a cancer of the plasma cells. The hallmark of any cancer is that one cell in the body grows out of control and when it’s myeloma, it’s a plasma cell. They live in the marrow, so we call it a bone marrow disorder, but the manifestations can be in the blood and outside the bone marrow.
The symptoms of myeloma, when those plasma cells grow too much, are often summarized by CRAB: high calcium (C), renal failure (R) or kidney dysfunction, anemia (A), and bone disease (B).
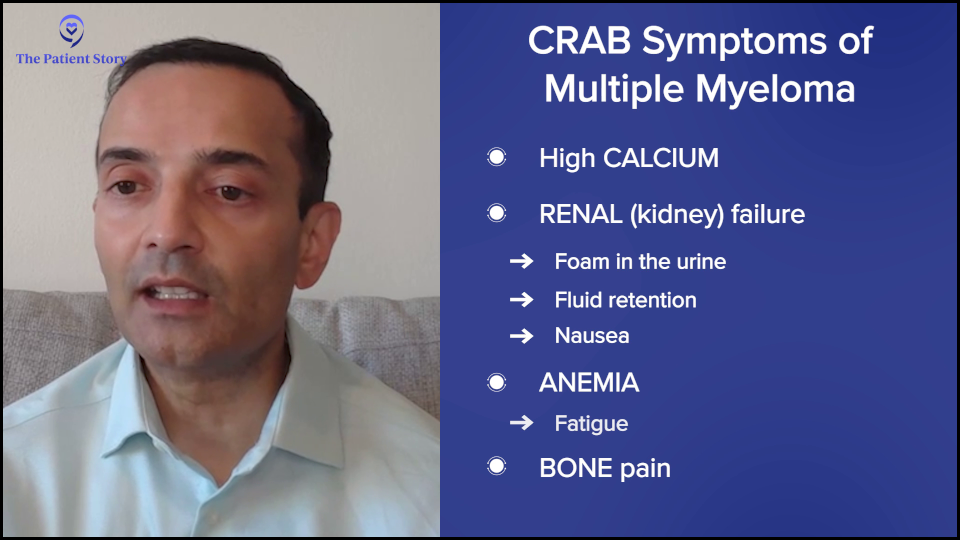
If they’re hypercalcemic, they can present with confusion or bone pain. Kidney failure can often be subtle. They may see foam in the urine. In advanced kidney failure, they may see fluid retention or nausea. Anemia often presents with fatigue, which is one of the more common symptoms.
Because it’s usually a slow-growing condition, it’s not something that pops up right away, but patients may gradually notice that they’re not able to walk as briskly or go up hills or stairs as readily. Everybody can have bone pain, especially as we get older, but it’s persistent, severe bone pain, and not something that comes and goes or fractures.

Treatment for Multiple Myeloma
Bryon: Dr. Chari, how do we treat it?
Dr. Chari: At a high level, cancers can be treated theoretically by chemotherapy, radiation, or surgery. In myeloma, there’s not a role for surgery other than to fix a fracture because it’s a bone marrow cancer. Radiation can be used for spot welding of pain at a particular site. But the mainstay of therapy is systemic or whole-body therapies.
Sometimes, people get concerned about the terms chemotherapy and immunotherapy. I would say any drug that’s used to kill cancer theoretically could be called chemotherapy because it’s killing cancer. A good example of that is steroids, everybody’s least favorite drug. Dexamethasone technically kills myeloma.

The classes of drugs in myeloma, which we can use in a newly diagnosed patient, are steroids, conventional chemotherapy (cyclophosphamide or Cytoxan, melphalan or Alkeran), immunomodulatory drugs (lenalidomide or Revlimid, pomalidomide or Pomalyst), proteasome inhibitors (bortezomib or Velvade, carfilzomib or Kyprolis), immunologic drugs (naked monoclonal antibodies, daratumumab or Darzalex, isatuximab or Sarclisa, CAR T-cell therapy, bispecific antibodies), and an XPO1 inhibitor (selinexor or Xpovio).
Those are the six classes of drugs and we can mix and match these based on efficacy and safety data, and how they will work and combine. For a newly diagnosed patient, the field has moved to, at a minimum, three drugs. Everybody gets steroids, at least at the beginning. Most people get lenalidomide (Revlimid), which is an oral drug, and CD38 antibodies, such as daratumumab (Darzalex) or isatuximab (Sarclisa).

There’s now a movement for younger patients and now even older patients to also get a fourth drug, which is a proteasome inhibitor, either bortezomib (Velcade) or carfilzomib (Kyprolis). The challenge there is that daratumumab (Darzalex), lenalidomide (Revlimid), and dexamethasone, which is called DRd or MAIA, is projected to give a five-year remission for the typical older patient even without a transplant.
To me, it’s not clear that every patient needs an extra drug because the fourth drug can come with additional side effects. But if it’s a fit older patient, certainly four drugs can be done. The other question is how long you need to continue. There are these movements of “down with dex.” Get the myeloma under control, but then drop it off, and then similarly drop the proteasome inhibitor.
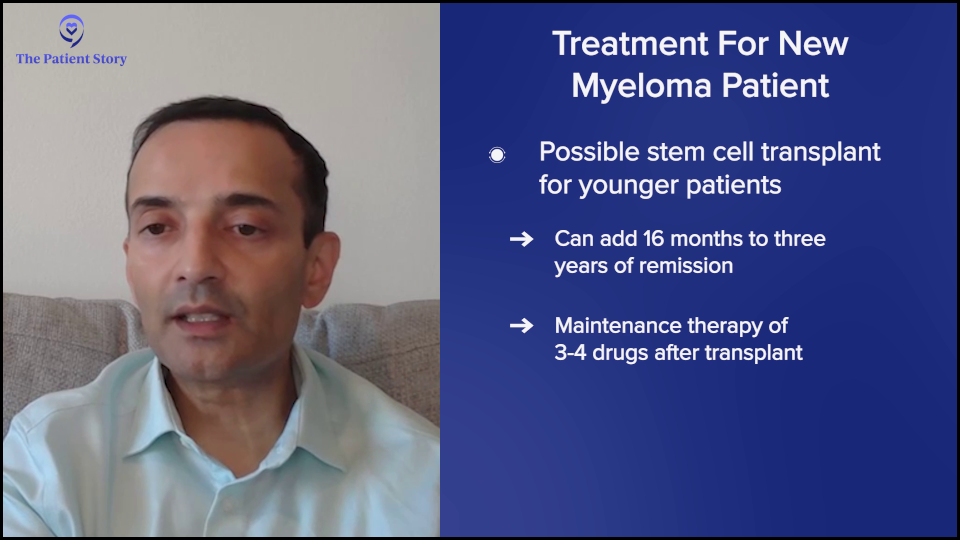
For younger patients, there’s the question of whether or not to do the transplant. It’s not wrong to do the transplant, particularly for high-risk patients. The value added is even higher. For the typical patient, transplant adds about 16 months of remission. For high risk, it can be even up to three years.
Some people have an aversion to transplant and are more interested in CAR T-cell therapy, but we need those data sets to read out that CAR T-cell therapy’s better than transplant. Whether or not you do transplant, you go to maintenance therapy typically of three to four drugs. Lenalidomide (Revlimid) is the easiest drug to maintain because it’s a pill. Some people also get daratumumab (Darzalex). Very rarely, we also do the other drugs.
We hope these remissions last a long time. Some of the quadruplets are now projected to last, believe it or not, 90 or 100 months, so eight to nine years, which is amazing.
But unfortunately, many patients will relapse. When a patient relapses, you could go back to any of those drugs on that six classes, except you can’t go to drugs that the patient’s no longer responding to. For example, if somebody was on lenalidomide (Revlimid) maintenance, you wouldn’t use that. If somebody was on lenalidomide (Revlimid) and daratumumab (Darzalex), you wouldn’t use those.
There are a lot of options and it’s impossible to go through them in a short time, but at a high level, the more high-risk the patient is, meaning the earlier or the more aggressive the relapse, the more you might want to consider CAR T-cell therapy because it’s now approved for one to three lines of prior therapy.
The slower and more well-behaved a myeloma is, you may be able to get away, for example, with a combination a CD38 monoclonal antibody, carfilzomib (Kyprolis), and dexamethasone, like daratumumab (Darzalex), carfilzomib (Kyprolis), and dexamethasone (DKd), or isatuximab (Sarclisa), carfilzomib (Kyprolis), and dexamethasone (Isa-Kd). You could also use the other drugs that we mentioned, like pomalidomide (Pomalyst). At a high level, that would be the first relapse. There’s now data to even potentially use belantamab (Blenrep), but it’s not yet approved. It’s an antibody-drug conjugate that could also be used in this space.

Lastly, for those who’ve had more than one relapse — they’ve had initial therapy, first-line therapy, and they’re now in second line and beyond — it’s the same principle. You can’t use drugs that the patient’s no longer responding to and, of course, you wouldn’t use drugs that the patient didn’t tolerate in the past. Beyond that, you could come up with any other combination. For example, you could do carfilzomib (Kyprolis), pomalidomide (Pomalyst), and dexamethasone (KPd) if you haven’t had those drugs before. You use all the drugs that you can.
Currently, bispecific antibodies, which are also exciting, require four or more lines of prior therapy, so that’s how the sequencing works. If you’re going to consider CAR T-cell therapy, it needs to be done before bispecific antibodies and antibody-drug conjugates because those will adversely affect the CAR T-cell therapy outcome. That’s a high-level overview of how we approach myeloma therapy.

What Roles Do Age, Gender, and Race Play in Multiple Myeloma Treatment?
Bryon: What role do demographics like age, gender, and race play in treatment?
Dr. Chari: We would love to personalize myeloma. Our closest example is translocation t(11;14), which was targetable by venetoclax (Venclexta, Venclyxto), but it was very complicated. Part of the reason is that even though patients did better with that drug, the studies were not always clear and I think it has to do with how complex myeloma is. It’s not just the genetic component. There are also a lot of important patient factors.
One of the unmet needs in myeloma is for the frail elderly patients, people who are 70-plus with a lot of medical problems. We have to do better for them. It’s great to have all of these amazing regimens, but if you can’t give them safely, then that’s a problem. Younger patients typically do better.
Concerning gender, we’ve seen pretty balanced outcomes. One thing is that CAR T-cell therapy with the rare Parkinsonism seems to be seen more in men than women, but that’s also because that’s where the demographics of Parkinson’s are, in that men get it more than women.

With race, the biggest thing is disparities in outcomes. Not everybody’s getting access to therapy. For example, when you look at the veterans’ hospital where everybody has access to therapy, you don’t see the differences in race-based outcomes. It’s when you take away equal access that you start to see differences. There are some studies suggesting that African-Americans may have better disease biology, so they may even have better outcomes with the same therapies if everybody’s getting access equally.
I would add that not just race, but anybody underserved, which includes economically disadvantaged and people in rural communities. We’re such a large country and if you live in a rural part of the country, you may not have access to some of the novel, exciting therapies. We have a lot of work to do in transposing all of these amazing outcomes and exciting drugs to all patients in the U.S. with myeloma and, honestly, globally, too.

Deciding on the Best Treatment Options
Bryon: Doctor, when I was diagnosed, I had a sit-down visit with my oncologist to discuss next steps. We talked about clinical trials, of course, but that’s not always the case. I decided at the time that I didn’t know enough to decide on the spot, so I said, “What is the standard line of treatment? I prefer to follow that and then see what happens.” How do you decide what treatment options are best for a patient?
Dr. Chari: Myeloma is not one of the most common tumors. The most common cancers are breast, colon, prostate, and lung. If you’re a patient who’s diagnosed in the community, chances are your oncologist is very experienced with those cancers, but possibly not as much with myeloma.
Studies show that outcomes are better for patients who are affiliated in some way with an academic center. I always tell my patients that they don’t get points for suffering. You don’t need to drive hours to come see me or get the same treatment you’re going to get close to home.
However, you should have a connection with an academic doctor who knows and keeps up with the latest data. Myeloma publications are like drinking from a fire hydrant. Every year, there are multiple, high-impact publications. As a community oncologist who’s treating all those other cancers, how can you possibly expect one person to be an expert at everything?
If you’re a patient, you want expert opinion, but the convenience of getting it close to home. At a minimum, get that academic opinion, hear about the options that are being proposed, which may or may not include clinical trials, and then make a decision.
Remember that today’s clinical trials are tomorrow’s standard of care. Every treatment that you’re getting today was because people like you did studies before you. We found that these newer therapies often displace the older therapies. It’s important to get your options, but also to recognize that some patients may not be eligible. If you have kidney failure or your counts are too low, you may not be eligible for studies, but it never hurts to have the options.
Benefits and Challenges of CAR T-cell Therapy
Bryon: A lot of your research has been focused on CAR T-cell therapy. Can you explain how it works and some of the challenges with T-cell therapies?
Dr. Chari: People ask why they got myeloma. The short answer is we don’t know, but the fact that most people get it in their 60s and 70s suggests that perhaps the immune system gets lazy as we get older. Maybe we all have a little bit of cancer when we’re younger, but our immune system gets rid of it. As the immune system gets lazy, we can lose that ability.
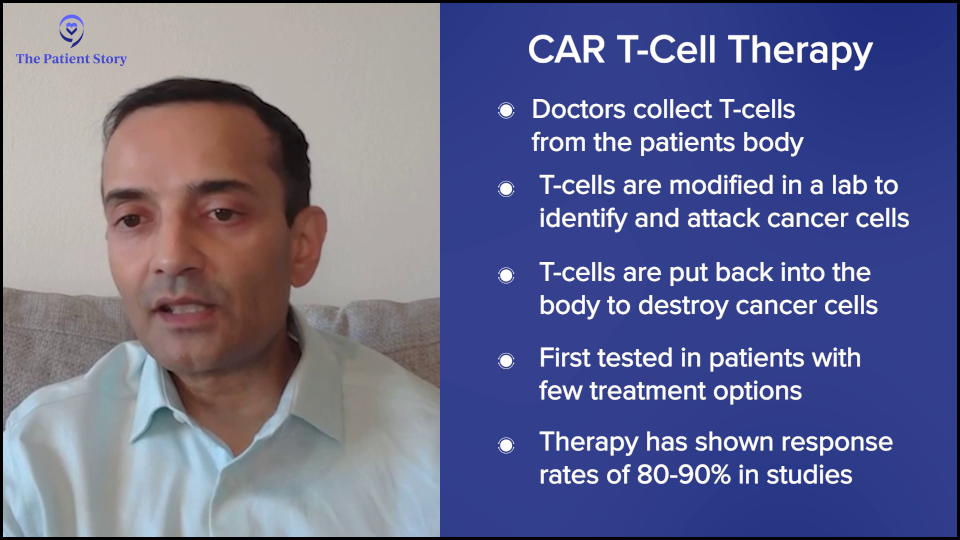
One way to deal with that is you can take somebody’s T cells out, genetically modify them, and put them in the patient. Essentially, these supercharged T cells can then kill the myeloma.
Initially, I have to say we were not too excited about this. As with all clinical trials of new therapies, we typically test them in people who have exhausted all available options because that’s the only way to do it. If you don’t know the side effects of something, you first start with patients who have very limited options.
When you take patients who are older and have been treated with many chemotherapy regimens, you don’t necessarily think the new therapies are going to work, but guess what? These CAR T-cell therapies are working like gangbusters.
When we put these supercharged T cells into patients, we’re getting responses of 80 to 90% lasting from one to three years in patients where you would historically expect — at best — a new exciting therapy to give a 20 to 30% response rate lasting from three to four months.
It’s a paradigm-shifting time. CAR T-cell therapies have taken patients who are almost hospice-bound and now put them in stringent complete remission, off therapy, and enjoying their lives.
Bispecific Antibodies vs. CAR T-cell Therapy
Bryon: Another area of research is bispecific antibodies. What advantages do bispecific antibodies offer over CAR T-cell therapies?
Dr. Chari: CAR T-cell therapy has to be very personalized. You have to collect T cells, genetically modify them, and put them back, so it’s personalized therapy. There’s what’s called vein-to-vein time, which is the time from collection to administration, and that can be anywhere from four to six weeks.
I would add that there’s also a brain-to-vein time. It’s from the day patients who are in the community are evaluated by their doctor and referred to an academic center, to when they get insurance approval and have their T cells collected.
The problem is that some patients don’t have the luxury of time. Whether it’s four to six weeks or several months, if your myeloma is taking off, you don’t have the luxury of waiting, so you need an off-the-shelf product that’s ready to go and this is where bispecific antibodies come in.
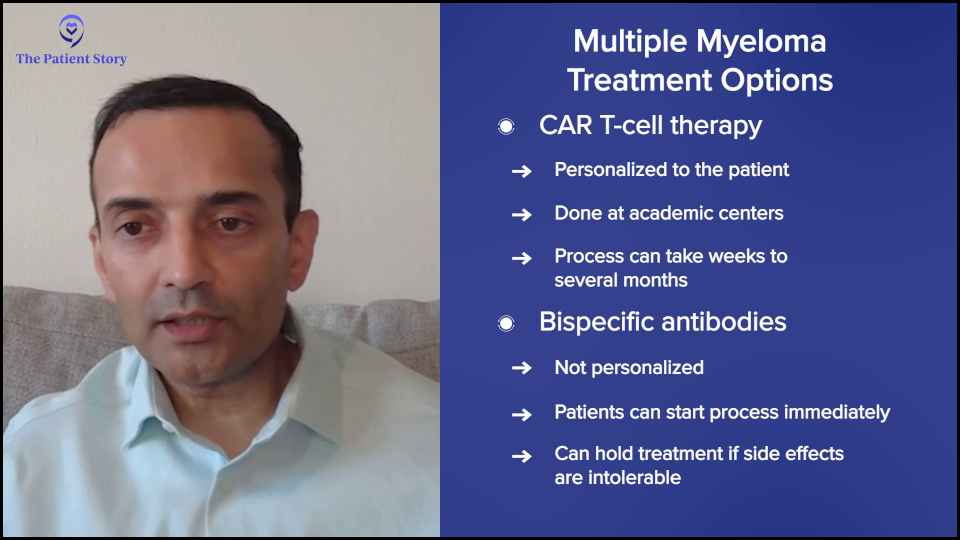
Bispecifics are like all other myeloma medications. If there’s a side effect, you can stop the drug and wait for the side effect to get better.
With CAR T-cell therapy, you administer it once it’s manufactured and after you put it all in, you don’t have control. You can’t take it back. If somebody has severe side effects, you just have to manage them. You can’t pause or reverse the process. In patients who have a lot of medical problems, you have a little bit more control over bispecifics than you do with CAR T-cell therapy.
Those are the two main differentiations: off-the-shelf and the ability to stop the therapy.
What are Trispecific Antibodies?
Bryon: Can you elaborate on the development and potential impact of trispecific antibodies in multiple myeloma patients?
Dr. Chari: CAR T-cell therapies and bispecific antibodies have historically targeted one antigen or one protein, which is ideally overexpressed on myeloma and not on other cells. The reason you want to do that is if you’re going to activate your T cell, whether with a CAR or bispecific, you don’t want them to attack your heart, kidneys, lungs, or normal parts of your body. You want those T cells to be like snipers and go after the cancer. We do that by selecting proteins that are overexpressed in myeloma but not in normal body parts.
Bispecifics bind your T cell, which is your immune cell, and your myeloma cell. With CAR T-cell therapy, the T cells are supercharged to kill one protein. Trispecifics are saying, “Why do we have to stick with one protein? Why not have the snipers go after two different proteins to increase the specificity?”
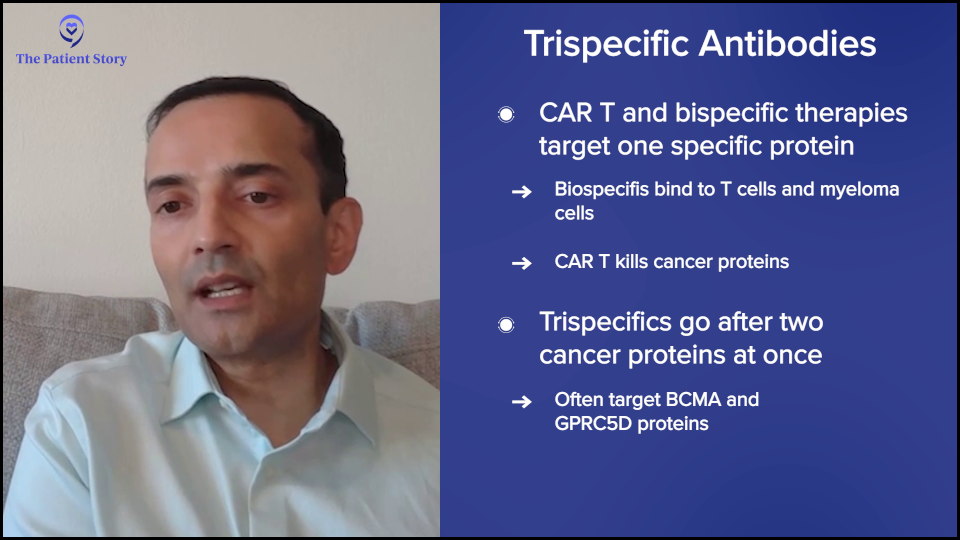
Even though we try to pick proteins that are overexpressed in myeloma, some of the other cells that express these will also be hit. For example, many people know about BCMA or B-cell maturation antigen. When you target that protein, you kill the myeloma cell, but you also kill some other immune cells, which can then increase the risk of infections.
The other big protein that we target is GPRC5D, which stands for G protein-coupled receptor class C group 5 member D. A mouthful, but it’s basically a protein that’s overexpressed on myeloma. But this protein can also be overexpressed in tissues that have a lot of keratin, like the nails and skin, so you see some of those side effects. It also causes oral side effects, like affecting taste and weight loss.
The trispecific is saying, “What if we target both? Let’s take the T cells and attack GPRC5D and BCMA at the same time. Will that decrease some of these side effects? Can you get even better responses?” It’s a nuclear war. When we go after one protein, the myeloma cell stops making that protein. By targeting two at the same time, it’s harder for the myeloma cell to bypass that.

Those are some of the theoretical benefits, which are possibly fewer side effects and hopefully more efficacy and better activity, but we’ll have to see. Preliminary data will be presented at the 2025 American Society of Clinical Oncology (ASCO) meeting and there’s a lot of excitement about this approach.
Another difference is when you give bispecifics and CAR T-cell therapy, after the first dose, sometimes you have massive T-cell activation. We call that cytokine release syndrome (CRS), which can present with fever and low blood pressure, often requiring hospital admission. By doing this dual targeting, will that make the safety profile better?
Lessons from Clinical Trials
Bryon: These novel innovative treatments that you mentioned have emerged over the past few years at a rapid pace. Let’s talk about clinical trials. There have been a few trials recently looking at different combination therapies, such as the AURIGA trial, MajesTEC-5 trial, and DREAMM-7 trial. What are researchers hoping to understand about these treatment options?
Dr. Chari: When we start with the patient journey, most people are getting an initial therapy of a combination of three drugs, now often four, then many people get a transplant. What happens after a transplant?

AURIGA Trial
Dr. Chari: If you’ve gone through initial chemotherapy and transplant and you’re still not what I call the A+ of myeloma — which means not only have we eradicated all the myeloma in the blood, the urine, and the bone marrow, but even the minimal residual disease — can we improve your outcome to get you to an A+?
In the AURIGA study, you had to be MRD positive after a transplant and could not have had a CD38 monoclonal antibody, like daratumumab (Darzalex) or isatuximab (Sarclisa), in your initial therapy. You could have had chemotherapy and a transplant, and you were still MRD positive. In the study, half of the patients got lenalidomide (Revlimid) alone and the other half had the addition of daratumumab (Darzalex) as a shot.
What that study showed is that the A+ conversion was improved, so more patients got MRD negativity, which, more importantly, translated into doubling of the remission duration. That’s now a strategy that could be used. Although it’s a randomized phase 2, not a phase 3, there’s a lot of excitement about trying to improve outcomes.

MajesTEC-5 Trial
Dr. Chari: Similarly, the MajesTEC-5 trial takes patients who are post-transplant and asks, rather than just doing lenalidomide (Revlimid) maintenance as a minimum, can you do better than that? One of the ways they’re trying to do that is by incorporating a BCMA bispecific known as teclistamab (Tecvayli), which is why it’s called MajesTEC-5. The TEC in MajesTEC refers to teclistamab.
This is not a randomized study. It’s a small study of only about 30 patients in two different arms, but it showed dramatic responses where the MRD negativity achieving A+ was markedly increased. Pretty much everybody achieved MRD negativity in this study.
Now, of course, we haven’t talked a lot about side effects, but these bispecifics can increase the risk of infections when you’re targeting BCMA. We’re going to need larger data sets to understand how to put getting an A+ into context with the potential risk of infections and needing preventative drugs and intravenous immunoglobulin (IVIG). It’s hard to argue against an A+ for 100 % of patients and so I think that’s where a lot of excitement is.

DREAMM-7 Trial
Dr. Chari: With the DREAMM-7 trial, patients have had an initial therapy, they may or may not have had a transplant, and they may or may not have had maintenance, but now the myeloma has come back. What can you do?
Historically, because a lot of patients are getting lenalidomide (Revlimid) maintenance after transplant, you can’t use lenalidomide (Revlimid) anymore. You have to move on. We could use daratumumab (Darzalex) and bortezomib (Velcade), and dexamethasone (DVd), but can we do better than that?
In this study, they took bortezomib (Velcade) and dexamethasone, but swapped out the daratumumab (Darzalex) with a new BCMA antibody-drug conjugate known as belantamab mafodotin (Blenrep), or bela for short. Basically, it’s a three-drug versus three-drug combination and everybody got Velcade-dex. The question being asked is belantamab (Blenrep) versus daratumumab (Darzalex). What that study showed was, surprisingly, belantamab (Blenrep) beat the pants off daratumumab (Darzalex). This was a marked improvement in the remission duration.

Now, this drug does come with eye side effects. It can cause dryness of the eye, irritation, and blurry vision. Most of them are reversible and sometimes they can hold the belantamab (Blenrep). This is a great option because if you can beat daratumumab (Darzalex), then why not?
The only limitation is that very few people will be in this population. These are typically going to be older patients. For most people who are younger with relapsed myeloma, you’re going to want to think about CAR T-cell therapy. If you give drugs, like belantamab (Blenrep) or BCMA bispecifics, you’re going to impair the CAR T-cell therapy outcomes down the road, which we know from some clinical trials.
For example, cilta-cel (Carvykti) gives a three-year remission. If you give a bispecific that goes to five months and an antibody-drug conjugate that goes to nine months, we don’t want to impair that amazing CAR T-cell therapy outcome.
For those who may not be eligible for CAR T-cell therapy— either they don’t want to do it, or they’re perhaps old and have a lot of medical problems — DREAMM-7 could be a good and exciting option. The other big difference is that it could be done in the community. You don’t need to go to an academic center to get belantamab mafodotin (Blenrep).
Why Should a Patient Consider a Clinical Trial?
Bryon: Why should a patient consider a clinical trial?
Dr. Chari: I was in New York for about 18 years, including the time when CAR T-cell therapy and bispecific antibodies came. I remember patients had exhausted all the therapies at that point. They’ve had the big five: lenalidomide (Revlimid), pomalidomide (Pomalyst), bortezomib (Velcade), carfilzomib (Kyprolis), and daratumumab (Darzalex). What do you do for someone like that?
We’ve had hospice conversations with some people, but let’s try this study. Those patients are now in their deepest and most durable remissions in their entire myeloma journey, despite having had all those drugs, which is a clear example. If you’ve run out of options, then you should be looking for new strategies. Many people would understand the need for clinical trials there. Even if you’re newly diagnosed or have had an early relapse, it’s also important to think about a clinical trial.
Back when I was a fellow, our initial therapy was two drugs, lenalidomide (Revlimid) and dexamethasone. How would we know that we need to do three drugs, then four drugs, a transplant, and maintenance? That’s been incremental learning from every clinical trial and that’s how medicine progresses. It’s always good to think about how you can get the best care for yourself and how you can advance the science for everybody.

Fears About Participating in a Clinical Trial
Bryon: The results of those clinical trials helped to determine what’s best for any given patient based on the data that comes out of trials. There are a lot of myths about clinical trials, as you know. One of the concerns we address with Myeloma Link is the fear that people will be receiving a placebo and getting no treatment or that the participants will become guinea pigs. Think about the Tuskegee syphilis study or the harvesting of Henrietta Lacks’ cells. What do you tell patients about these fears?
Dr. Chari: Fear is understandable, especially when you’re dealing with a diagnosis of cancer. When I was a medical student, we had a class called “Is It Cancer and Can I Still Have Sex: The Two Questions Patients Had, But Were Afraid to Ask.” What you learn is that 50% of the information during that first cancer visit goes in one ear and out the other because not only are they dealing with the diagnosis and the intellectual questions, but also the emotional aspect. It’s always good to have a family member or friend with you to listen in on all of your conversations, so that they can help catch some of the things you might have missed or record the visit, if your provider allows.
It’s also important to understand that because of the Tuskegee syphilis study, Henrietta Lacks, and all of these other stories, there are a lot of protections built in. As an investigator, you can’t simply say that you want to test a new drug.
You have to write a protocol. You have to write a consent form, which independent doctors review on several committees. The FDA has to oversee it. If it’s sponsored by a company, the company has to oversee it. There are a lot of checks and balances within the institution, the pharmaceutical company, and the FDA. There are three different major stakeholders.
As a patient, you have to ask: What’s the question being asked? For example, it’s unethical to take somebody with advanced myeloma, who has active disease, and assign them to a placebo. That would never pass all of those various committees.

Where you might see a placebo is if you’re not sure if you need an extra drug. If the combination of three drugs is doing pretty well and you want to add a fourth drug, you might have to do a placebo arm. There is a placebo effect. When people take a drug, they’re going to feel better and think they’re getting a better outcome.
In my experience with myeloma, the placebo-based studies are very few and far between. Probably the only time you see that is in maintenance, where you don’t know if you should be doing something preventively or wait for the cancer to act up. Or perhaps in smoldering myeloma, where you don’t have symptoms and you’re trying to prevent the cancer, you might think about doing something like that. But even with those, the field is moving.
In cancer, the concern that you’re going to be a guinea pig is typically because of an admitted lack of knowledge. Otherwise, if we knew the answer, we wouldn’t be doing it, so there is an inherent unknown. We put a lot of safeguards in to mitigate that.

Managing Side Effects of Multiple Myeloma Treatment
Bryon: Let’s talk about managing treatment side effects. What are some of the common side effects people experience from treatment and what can be done to mitigate them?
Dr. Chari: This is a complicated question because it is very drug-specific and therapy-specific.
If we’re talking about steroids, everybody’s least favorite drug, the possible side effects include irritability, sleep, heartburn, and fluid retention. You can try to lower the dose and, if possible, even stop it. You could also take a sleep medication.
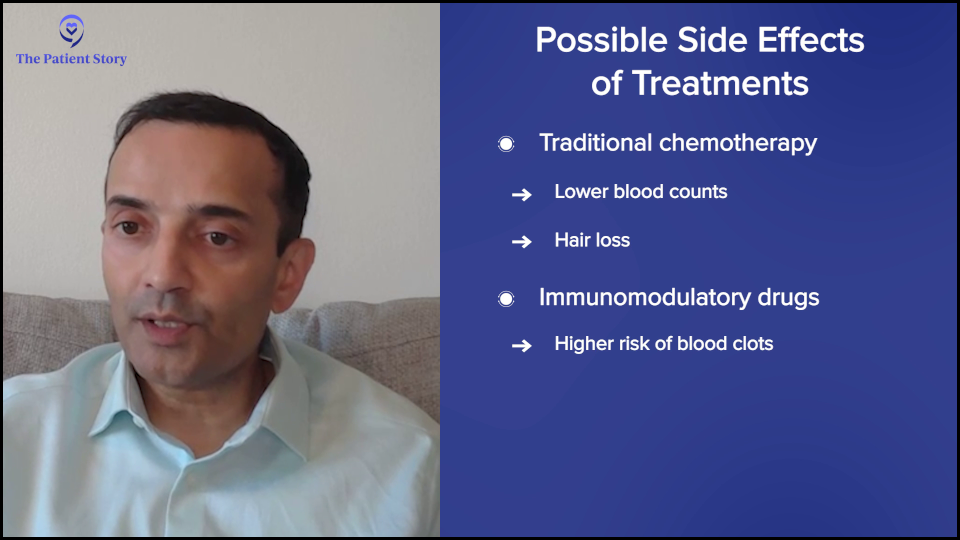
Conventional chemotherapies often cause a lowering of blood counts because they kill the myeloma cells. We’re talking about drugs like melphalan (Alkeran), which is part of the transplant. There’s some interest in cooling caps for reducing hair loss that are showing some activity at our institution.
For the IMiDs (immunomodulatory drugs), like thalidomide (Thalomid), lenalidomide (Revlimid), and pomalidomide (Pomalyst), they typically increase the risk of blood clots, so it’s important to take an aspirin to prevent those. If you’re on certain other drugs, have a history, or have a high risk, you might want to take something stronger.

With proteasome inhibitors like bortezomib (Velcade), carfilzomib (Kyprolis), and ixazomib (Ninlaro), there’s a risk of shingles, so you would want to take a preventative pill. Sometimes, bortezomib (Velcade) can cause neuropathy, and carfilzomib (Kyprolis) can cause heart issues, so you have to monitor blood pressure. Immunotherapy drugs can also increase the risk of infection, so you have to monitor that.
CAR T-cell therapy and bispecific antibodies can cause cytokine release syndrome, which has to be monitored and treated. With bispecifics, there is also the risk of infections and oral toxicity, which you have to do supportive care, like IVIG, or modify the dose.
When Would a Doctor and Patient Consider Switching Treatment?
Bryon: At what point would side effects lead to considering switching a patient from one treatment to another?
Dr. Chari: This is always a balance between efficacy and safety. What kind of myeloma is this? How well is it controlled? How bad are the side effects? If somebody has very favorable myeloma with very little disease, you’re not going to tolerate many side effects. If you have very difficult-to-control myeloma or very high-risk myeloma with multiple genetic abnormalities, there might be more willingness to take on side effects.
Ultimately, you have to give it a shot. This is why oncology is a specialty. You have to know this kind of risk-benefit balance and walk this tightrope. That’s why we have frequent visits and labs so that you can make treatment decisions. Am I happy with the myeloma control? How bad are the side effects that this patient’s having? Is the patient willing to continue with some modifications of dose and schedule or medications to offset side effects?

Quality of Life Concerns for Younger Patients
Bryon: Multiple myeloma is generally diagnosed in older patients. What are some of the quality of life considerations that you discuss with patients who are younger than 50?
Dr. Chari: My youngest patient was diagnosed below 18 and the oldest is over 100. It’s a very heterogeneous disease, so it’s hard to do a one-size-fits-all. There’s a movement in all of medicine, specifically in oncology, to not use a chronological age and to focus on how healthy that person is. Some 70-year-olds are going to be way healthier than 50-year-olds who might have multiple other medical problems.

For older patients, you have to look at their other medical issues. For young patients, you’re trying to walk that tightrope between preventing the disease from coming back and allowing them to live their lives. It’s important to talk about fertility preservation, maybe banking sperm or freezing eggs and embryos if possible, understanding what may or may not be feasible depending on the pace of the disease. Ultimately, it’s what we do in oncology. You have to personalize the therapy for each patient.
Promising Treatment Developments
Bryon: What are the most promising developments in multiple myeloma treatment that you foresee in the next few years?
Dr. Chari: The first is moving therapies that are currently in advanced myeloma to earlier and earlier lines of therapy. Initially, CAR T-cell therapy was for heavily treated patients; now it’s approved for patients who have had one to three prior lines. There are clinical trials that may lead to their approval in newly diagnosed patients and perhaps even smoldering myeloma. Similarly, bispecific antibodies are making their way up, showing 100% response rates and MRD negativity in earlier lines of therapy.

We also need new combination therapies. Let’s say somebody has had CAR T-cell therapy and bispecific antibodies. What do we do then? When you beat up all the T cells with all these therapies, you need non-T-cell-dependent strategies. Some new targets are promising, like bromodomain inhibitors and trispecifics that target different antigens.
Then we talked about dual CARs, triple CARs, and newer CARs that don’t have the same safety issues, some data that maybe we don’t see Parkinsonism, improving on existing therapies and moving them earlier, and having new targets.
Importance of New Immunotherapies
Bryon: How do you envision the integration of novel immunotherapies into standard treatment protocols for multiple myeloma?
Dr. Chari: Thankfully, the health authorities across the Atlantic, both our FDA and Europe, have allowed MRD negativity. The MAIA study had a median age of 73, who received daratumumab (Darzalex), lenalidomide (Revlimid), and dexamethasone (DRd). If you start at 73, those patients are going to get a five-year remission. If you’re going to try to improve on that combination, imagine how long you’re going to have to wait before hundreds of patients are accrued and randomized before we find out if they’re better.
The MRD negativity is a huge advancement in earlier lines of therapy because then, MRD negativity should translate into progression-free survival, which is how long the remission lasts. Ideally, that could lead to living longer. Sometimes people are looking for overall survival improvement, but as you get to newer and newer diagnoses of myeloma or less heavily treated, the number of drugs that we have to rescue myeloma can make it very difficult to show that the initial therapy is going to translate into living longer. MRD negativity is going to be a big one.

Honestly, we need these clinical trials, like CAR T-cell therapy versus transplant, which I think is super exciting. I don’t think anybody wants to go through a transplant voluntarily. If we can do new therapies that don’t cause hair loss, nausea, vomiting, diarrhea, weight loss, and fatigue, by all means, we should get rid of it.
But we need studies to show not just that they’re better, but remember, we also need to think about long-term side effects. Because myeloma patients are living longer, there’s an increased risk of getting other cancers, so we need to follow patients long enough to know that not only is the initial benefit there, but there are no unexpected longer-term side effects.
Advice for Newly Diagnosed Patients
Bryon: Dr. Chari, as we wrap up, if there’s one thing you want someone newly diagnosed with multiple myeloma to know about their treatment options, what would it be?
Dr. Chari: My passion is the personalization of therapy. No two patients are alike. You can read about a clinical trial, you can go to a patient support group, and you can talk to a friend, but that doesn’t mean that’s the right treatment for you.

There are patient factors to consider, like age, kidney function, heart history, diabetes, and neuropathy. There’s the disease, like your ISS stage and genetics. Do you have myeloma coming out of the bone marrow or extramedullary disease (EMD)? There are treatment considerations. What side effects are you willing to accept? Can you take time off work? Do you have a caregiver? What therapies have you had?
I see a lot of patients who come in the door so anxious that they just want me to give an immediate answer as to what I would recommend. You have to put all of those factors that I mentioned together. I need to know your medical history, your cancer, and what you want as a patient. Then I can give you an answer. But if you’re just asking for an answer, you’re not getting the best care, and that includes the options for clinical trials.
I would encourage everybody to speak to a myeloma consultant at an expert medical center and make sure that all of these factors are being considered to personalize a therapy for you.

Conclusion
Bryon: Dr. Chari, thank you so much for sharing your insights.
We hope this discussion has provided you with valuable information. We encourage you to follow or continue following the latest advancements in the field and to discuss your options with your healthcare providers. Please check out Blood Cancer United’s Myeloma initiatives and support for patients and caregivers.
Stephanie: Thank you so much, Bryon and Dr. Chari, for leading this incredible conversation. We’re so glad that you could be a part of this discussion about promising clinical trials in multiple myeloma. We hope that this leaves you with new information, fresh hope, and a reminder that you are not alone as you’re dealing with this.
Every patient and care partner deserves to understand all of their options. Clinical trials are not just for someone else. They’re not a last resort. They can open doors to the latest in treatments and care. It’s all about having the conversation with your team to know your options. It may not be the right one for you, but at least you have that at your fingertips.
If you need any support, don’t hesitate to reach out to The Patient Story or Blood Cancer United. They have a Clinical Trial Support Center that offers free one-on-one support for clinical trial navigation, which happens even before the clinical trial. They can help you figure out the questions beforehand, including which clinical trial might be the right one for you. They also have a patient community and peer-to-peer support.
Remember, your voice and story matter. Keep advocating for yourself. Don’t be afraid to ask questions. Do your research. Talk openly with your team. Be curious. We hope to see you again at another conversation. Take good care.
We would like to thank Blood Cancer United (formerly The Leukemia & Lymphoma Society) for their support.
Visit their Multiple Myeloma Overview for information and a list of resources to help you navigate myeloma.
They offer free resources like their Information Specialists, who are one free call away for support in different areas of blood cancer.


Multiple Myeloma Patient Stories
Clay D., Relapsed/Refractory Multiple Myeloma
Symptoms: Persistent kidney issues, nausea
Treatments: Chemotherapy (CyBorD, KRd, VDPace), radiation, stem cell transplant (autologous & allogeneic), targeted therapy (daratumumab), immunotherapy (elotuzumab)
...
Melissa V., Multiple Myeloma, Stage 3
Symptom: Frequent infections
Treatments: IVF treatment & chemotherapy (RVD) for 7 rounds
...
Elise D., Refractory Multiple Myeloma
Symptoms: Lower back pain, fractured sacrum
Treatments: CyBorD, Clinical trial of Xpovio (selinexor)+ Kyprolis (carfilzomib) + dexamethasone
...
Marti P., Multiple Myeloma, Stage 3
Symptoms: Dizziness, confusion, fatigue, vomiting, hives
Treatments: Chemotherapy (bortezomib & velcade), daratumumab/Darzalex, lenalidomide, revlimid, & stem cell transplant
...
Ray H., Multiple Myeloma, Stage 3
Symptoms: Hemorrhoids, low red blood cell count
Treatments: Immunotherapy, chemotherapy, stem cell transplant
...






