Clinical Trials: Everything Cancer Patients Should Know
You may have heard about “clinical trials” or “clinical research” from your doctor or read it in an article. The term, itself, is technical-sounding. Here’s more about what they actually mean – in “human terms” – in the cancer space, with examples from actual patient stories (scroll below).
Clinical trials are studies that are key to advancing scientific development. They offer researchers an opportunity to understand the potential impacts of new drugs and give patient’s access to medicine that may not have been available to them otherwise.
What Are Clinical Trials?
Clinical trials are a stage in medical research that tests the effectiveness of a treatment on human patients. The FDA allows researchers to conduct clinical trials once a treatment has shown promise in animals. Clinical trials can be used to find treatments that are better at helping a medical condition, or to determine if a new type of treatment is safer than the current standard of care.

At the minimum, they provide a very comprehensive approach for how we treat patients because patients have to be under such great scrutiny, and there’s a whole team that supports how they conduct the clinical trial that patients have and get more attention than they would ever get in any other way.
Dr. Rafael Fonseca
What Do Clinical Trials Study?
Clinical trials test new interventions -meaning treatments like drugs, or other procedures- that have the potential to be more effective or safer than the current standard of treatment for a disease or condition. Researchers don’t yet know if the intervention used in a clinical trial works, and many treatments never make it past the clinical trial stage.
Without clinical trials, doctors would have no way of knowing if a better treatment exists for a disease. Clinical trials can result in new treatments or even cures for diseases that were previously considered terminal.
It’s important that patients understand that clinical trials aren’t a magic bullet — ultimately, only a small percentage of drugs in clinical trials will receive FDA approval.
The keyword is “pursuing” because we don’t know whenever you’re testing something new. You hope it works.You don’t know if it will, and you don’t know if it potentially could also be more toxic.
Dr. Rafael Fonseca

Are Clinical Trials Safe?
When you elect to participate in a clinical trial, you will have the risks of the trial explained to you. Potential risks include uncomfortable treatment, treatment that is not better than the current standard of care, and the fact that you may end up in a control group (group that does not receive the experimental treatment, instead receiving a placebo or typical existing treatment).
In the past, clinical trials were sometimes unsafe and unethical. Now, clinical trials must adhere to very strict standards that protect patients. In 1978, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research created The Belmont Report, a set of fundamental ethical principles and guidelines that assist researchers in resolving ethical principles when conducting research involving human subjects.
Modern-day clinical trials require explicit consent from the patient. Doctors will never involve you in a clinical trial without your consent, and you are allowed to end your participation in a clinical trial at any time.

We as physicians are trained to help them make that decision. My job is to help them make a decision that they will be comfortable with and that there won’t be any regrets later on…
Dr. Farrukh Awan
Clinical trials must also adhere to strict schedules. While this may mean your appointments are longer or not as flexible, it also means that you will know exactly when and for how long you are receiving treatment.
There’s a high degree of rigor. We can’t just go around and say, “Oh, I’m going to see you next week instead of this week.” If the clinical trial says something, we try to adhere to that as much as possible so that we have the high quality and the consistency of the data. You would know about it.
Dr. Rafael Fonseca

What Are the Main Phases of a Clinical Trial?
Clinical trials can go through up to four rigorous phases of testing to ensure that they are safe for the general population. Only about 33% of all clinical trial drugs make it through Phase II.
Phase I
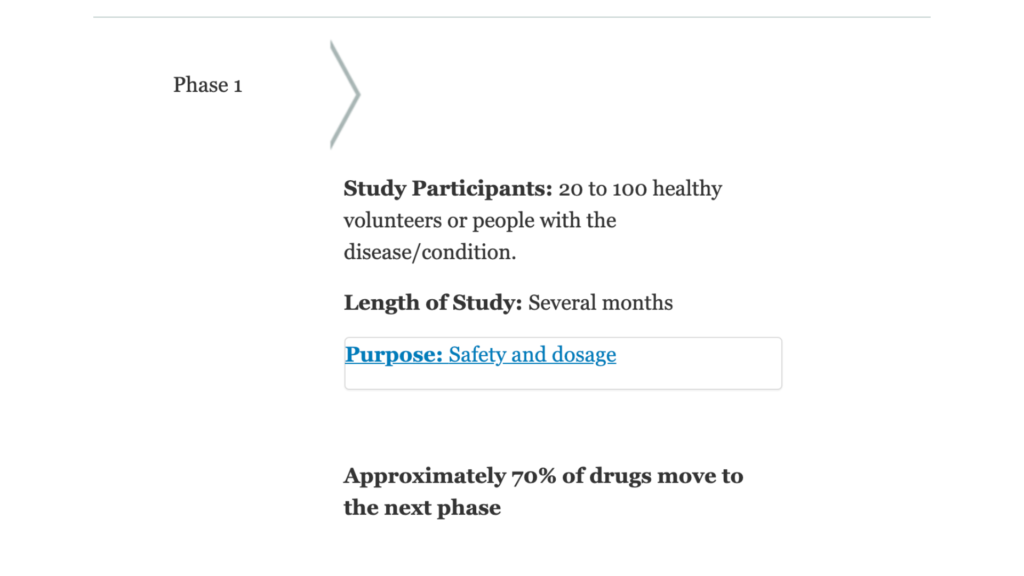
Phase I trials test drugs that often have never been administered to humans. The purpose of a Phase I trial is to determine whether the new intervention has the potential to help more than harm. Researchers use Phase I clinical trials to find out what a safe human dosage is.
Study groups in Phase I trials are usually very small. Participants are closely monitored for side effects. Phase I trials are not testing the effectiveness of a treatment — they are primarily focused on how safe the treatment is for humans.
Phase II

Interventions move on to a Phase II trial when they are determined to be safe in Phase I. The goal of a Phase II clinical trial is to see if the drug is effective at treating the target disease in humans.
Like Phase I trials, Phase II trials do not use placebos. Instead, participants either all receive the same dose, or receive a range of doses that researchers determined safe in Phase I in order to determine the optimal effective dose. Because more people are involved in Phase II trials, researchers will likely see side effects that did not occur in Phase I.
Phase III

If a drug shows promise in a Phase II clinical trial, it moves on to Phase III. Phase III trials test whether a drug or treatment is better than the current standard of care. For example, a new type of chemo might effectively kill cancer cells in Phase II — but does that mean it works better than the drug that is already used to treat that type of cancer?

Let’s say you take Drug A, and A is the standard of care. But, we think adding drug B might be good. If you do a Phase III trial, a frequent way of doing it is that everyone is going to get Drug A. That’s the standard of care… But then by a flip of a coin, we’re going to have half of the patients take Drug B, and the other half will take A. Patients know this. It has to be a very explicit discussion.
Dr. Rafael Fonseca
Placebos are used in Phase III trials, but patients are not given an inactive treatment if a treatment that already works for the disease exists. Phase III trials are often very large, taking place concurrently at medical facilities across the country or world.
Phase III clinical trials are often the last step before new drugs are submitted for FDA approval. After a Phase III trial, drugmakers will submit a new drug application (or NDA) to the FDA. The FDA can then accept the application, or return it with a request for further study.
Phase IV
Once a drug is approved by the FDA, it may enter a Phase IV trial. Phase IV clinical trials look at the effects of treatments over a longer period of time, often several years. Drugs in Phase IV trials have already been proven safe and effective — there is little risk to the patient in a Phase IV trial. Instead, the point of Phase IV trials is to help researchers know more about the long-term effectiveness and side effects of the treatment.
And for those who participate in trials, thank you. It is only because of those trials that we’re able to advance the science.
Dr. Rafael Fonseca

Note: Several quotes from this article come from The Patient Story’s interview with Dr. Raphael Fonseca, a practicing hematologist and myeloma specialist with over three decades of experience. To watch the interview click below.
What are the FDA’s Drug Approval Processes?
According to the FDA, “Over the past three decades, Congress has established five programs aimed at expediting patient access to important drugs that treat serious or life-threatening conditions.”
These five programs are:
- Fast Track designation – designation isgranted if there is an unmet medical need, and a drug’s data thus far shows promise.
- Breakthrough Therapy designation – designation can be given to drugs that show a significant improvement over therapies or drugs that are currently available.
- Regenerative Medicine Advanced Therapy designation (RMAT) – for getting regenerative medicine therapies (i.e., stem cell transplants) with the potential of meeting an unmet need for a serious condition on the market quicker.
- Priority Review – designation is opposed to the FDA’s standard review. Many of the new treatments the FDA approves fall under standard review. This is a process that usually takes about 10 months for review. With Priority Review, the goal is six months. This is for treatments that have shown proven, significant improvements for a serious condition.
- Accelerated Approval – a little different than the other four programs. It allows a drug to be approved based upon what’s called a “surrogate endpoint.” That’s medical jargon for “a blood test” or “a CT scan” that shows improvement that proves the drug is working. For example, if you had a tumor and were on a drug in the Accelerated Approval program, and the tumor was shrinking every few weeks, that might prove the drug was working without having to wait to see how you feel or how long the treatment extends your life since those things can take years to find out. Source
If a drug or therapy has one of these designations, it could mean that the FDA likely sees that it has the potential to fill a need. It can be helpful to search these designations for new and upcoming treatments that could meet your specific needs.
Clinical Trial Resources

What Is a Clinical Trial Really?
There are so many myths surrounding clinical trials. To bring some clarity to an often confusing, myth-riddled topic, The Patient Story and The Leukemia & Lymphoma Society (LLS) have teamed up to discuss what a clinical trial is, the phases of a clinical trial and how to figure out the logistics of paperwork, tests/scans and finances.
Clinical Trials Patient Stories
Learn about cancer patient’s experiences with enrolling and participating in clinical trials from real-life patients.
Breast Cancer
Abigail J., Metastatic Breast Cancer, HER2-low, PIK3CA+
Symptoms: Back and leg pain, lump in breast
Treatments: Surgery, chemotherapy, radiation, CDK4/6 inhibitors
Cervical Cancer
Sara I., High-Grade Serous & Clear Cell Carcinoma, Stage 3A
Symptoms: Random sharp pains, unrelated scan showed ovarian cyst
Treatments: Debulking surgery, chemotherapy (carboplatin & paclitaxel), PARP inhibitors (clinical trial)
Heather M., Epithelial Ovarian Cancer, Stage 2
Symptoms: Extreme bloating, pinching pain in right side of abdomen, extreme fatigue
Treatments: Surgery (total hysterectomy), chemotherapy (Taxol once a week for 18 week, carboplatin every 3 weeks), concurrent clinical trial (Avastin) every 3 weeks
Kidney Cancer
Bill P., Kidney Cancer (Papillary Renal Cell Carcinoma), Stage 3, Type 1
Symptoms: Kidney stone, lower back pain, sore/stiff leg, deep vein thrombosis (DVT) blood clot
Treatment: Nephrectomy (surgical removal of kidney and ureter)
Leukemia
Sean R., Chronic Lymphocytic Leukemia
Symptom: No apparent symptom; went to ER for unrelated shoulder pain
Treatment: Clinical trial (ibrutinib & venetoclax)
Michele N., Chronic Lymphocytic Leukemia
Symptoms: Slow healing, scalp infection, enlarged lymph nodes
Treatments: Clinical trial of ibrutinib, fludarabine, chlorambucil and rituximab; acalabrutinib
Mary Clare B., Acute Myeloid Leukemia (AML)
Symptoms: Extreme fatigue, upset stomach, bad & persistent headaches
Treatments: Chemotherapy, radiation, bone marrow transplants
Jeff F., Chronic Lymphocytic Leukemia
Symptoms: Fatigue, night sweats
Treatment: Clinical trial (ofatumumab)
Lung Cancer
Shyreece P., Non-Small Cell Lung Cancer, ALK+, Stage 4 (Metastatic)
Symptoms: Heaviness in arms, wheezing, fatigue
Treatments: Chemotherapy (carboplatin, pemetrexed, bevacizumab), targeted therapy (crizotinib, alectinib)
Melanoma
Multiple Myeloma
Elise D., Refractory Multiple Myeloma
Symptoms: Lower back pain, fractured sacrum
Treatments: CyBorD, Clinical trial of Xpovio (selinexor)+ Kyprolis (carfilzomib) + dexamethasone
Donna K., Refractory Multiple Myeloma
Symptom: None; found through blood tests
Treatments: Total Therapy Four, carfilzomib + pomalidomide, daratumumab + lenalidomide, CAR T-cell therapy, selinexor-carfilzomib
Non-Hodgkin’s Lymphoma
Stephanie R., Mantle Cell Lymphoma (MCL), Stage 4
Symptom: Elevated white blood cell count
Treatments: 6 months of rituximab + ibrutinib, 4 cycles of hyper-CVAD chemotherapy
Robyn S., Relapsed Diffuse Large B-Cell Lymphoma (DLBCL), Stage 2E
Symptoms: Enlarged lymph nodes
Treatments: Chemotherapy: R-CHOP, R-ICE, intrathecal, BEAM; autologous stem cell transplant, head and neck radiation, CAR T-cell therapy
Richard P., Diffuse Large B-Cell Lymphoma (DLBCL), Stage 4
Relapse Symptoms: Swelling in leg, leg edema Treatments: R-CHOP chemotherapy, clinical trial (venetoclax-selinexor)
Jason W., Mantle Cell Lymphoma (MCL), Stage 4
Symptoms: Hives, inflamed arms
Treatments: Calabrutinib, Lenalidomide, Rituxan
Cherylinn N., Mantle Cell Lymphoma (MCL), Stage 4
Symptom: None
Treatments: R-CHOP chemotherapy, rituximab
Ovarian Cancer
Sara I., High-Grade Serous & Clear Cell Carcinoma, Stage 3A
Symptoms: Random sharp pains, unrelated scan showed ovarian cyst
Treatments: Debulking surgery, chemotherapy (carboplatin & paclitaxel), PARP inhibitors (clinical trial)
Heather M., Epithelial Ovarian Cancer, Stage 2
Symptoms: Extreme bloating, pinching pain in right side of abdomen, extreme fatigue
Treatments: Surgery (total hysterectomy), chemotherapy (Taxol once a week for 18 week, carboplatin every 3 weeks), concurrent clinical trial (Avastin) every 3 weeks
Sarcoma
Alicia B., Desmoid Tumor, Stage 4
Symptom: Lump in right armpit
Treatments: Chemotherapy, radiation, targeted therapy, clinical trials, surgery, including forequarter amputation
Clinical Trials Experts
Discover medical professionals and experts thoughts and opinions on clinical trials.
The Doctor Talk w/ Dr. Gasparetto & Dr. Richter
Role: Dr. Cristina Gasparetto (Duke) and Dr. Joshua Richter Mount Sinai discuss latest relapsed refractory multiple myeloma treatments
Saad Z. Usmani, MD
Dr. Saad Usmani, Chief of Myeloma Service at Memorial Sloan Kettering, talks about CAR T-cell therapy, bispecific antibodies, novel therapies and combination therapies.
Polycythemia Vera (2022)
In segment 2 of our conversation with Srdan Vertsovsek, MD, PhD of MD Anderson, he shares the latest on research for polycythemia vera or PV treatments.
Additional Resources for Clinical Trials
- https://clinicaltrials.gov – Here you can search your condition and see the status of clinical trials for it. Look for ones that say “recruiting.” Even if you don’t understand all the jargon, you can always present it to your doctor and see if they think it’s a viable option.
- https://www.drugs.com/newdrugs.html – If you’d rather not be part of a clinical trial and want something that the FDA has already granted full approval, you can search for your condition here. This is a constantly updated list of newly approved treatments. Simply search for your condition, and you can read the press release (or show it to your doctor) about the new drug.



























