Navigating the Latest Metastatic Colorectal Cancer Treatments and Clinical Trials
Edited by:
Katrina Villareal
Please watch the above video replay then take our program survey.
Allison Rosen, a colorectal cancer patient advocate, talks with leading oncologist Dr. Cathy Eng from Vanderbilt-Ingram Cancer Center. Gain valuable insights into the latest advancements in the treatment of metastatic colorectal cancer and a deeper understanding of the current treatment landscape, including the most recent FDA-approved therapies that are making a difference.
Learn how biomarkers are revolutionizing the approach to treating metastatic colorectal cancer, potentially leading to more personalized and effective treatment plans. Discover emerging therapies and the most promising clinical trials available, offering new hope and options for patients. Equip yourself with the knowledge to collaborate effectively with your medical team, ensuring the best possible outcomes and quality of life.
We’d like to thank our promotional partners for their continued support of our programs. Please visit the Colon Cancer Coalition and Colontown to learn more about the important work they are doing.


The Patient Story retains full editorial control over all content. This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider for treatment decisions.
- Introduction
- Latest Treatment Options for Metastatic Colorectal Cancer
- Trending to More Personalized Treatment
- Biomarkers (“Molecular Markers”)
- Choosing Among Different Treatment Options
- Emerging Clinical Trials for Metastatic Colorectal Cancer Patients
- Importance of Participating in Clinical Trials
- Final Takeaways
Introduction
Stephanie Chuang, The Patient Story Founder
Stephanie Chuang: I’m a blood cancer survivor and the founder of The Patient Story, which I started out of my own experience. I had so many questions and all I found was medical jargon and outdated statistics.
Our goal at The Patient Story is to help patients and care partners navigate life after a diagnosis. What does this mean in human terms? We do this primarily through in-depth patient stories and educational programs. We hope to help connect patients and families to information about topics like self-advocacy and the latest treatment options, which is especially important in colorectal cancer. It makes a difference how early someone can get a diagnosis, how much people know about what’s available to them, and what questions they can ask their healthcare providers.
The Patient Story retains full editorial control of the content. We also want to thank our promotional partner, the Colon Cancer Coalition.
While we hope you walk away with more knowledge, this discussion is not a substitute for medical advice so please consult with your healthcare team.
It’s my pleasure to introduce Allison Rosen, a very well-known and respected individual in the colorectal cancer advocacy space and someone I’m lucky to call a friend. Thank you so much, Allison, for joining us.


Allison Rosen, Patient Advocate
Allison Rosen: I’m a 12-year stage 2C colorectal cancer survivor and a very passionate patient advocate. My treatment consisted of surgery, radiation, chemotherapy, more surgery, and ultimately, a permanent ostomy.
I wanted to meet other young people like me who were diagnosed with colorectal cancer because I felt very alone when I was first diagnosed. When I was being treated at MD Anderson, I asked to talk to another young adult. She was very active in advocacy and introduced me to the advocacy space. Now I volunteer with a lot of national and local advocacy organizations. I get to work with amazing clinicians, researchers, and doctors to help provide the patient perspective.
Dr. Cathy Eng: You are so fortunate to be diagnosed with stage 2 because, unfortunately, most young adults are diagnosed with a much more advanced stage. Stage 2 is a very unique setting because it’s extremely early with regard to prognosis. For the majority of patients, surgical resection is curative. I’m so glad to have you here with us today.
Allison: Thank you. It’s interesting because the reason why I’m so active is because I’m alive. I have a purpose and my mission is to be a part of things like this educational program to talk about what is out there, so people who may not have access to what I had access to can get current information and the best possible treatment.

Dr. Cathy Eng, Medical Oncologist
Allison: Dr. Cathy Eng is the David H. Johnson Endowed Chair in Surgical and Medical Oncology at the Vanderbilt-Ingram Cancer Center. She’s also the co-leader of the Gastrointestinal Cancer Research Program, co-director of GI Oncology at Vanderbilt, the executive director of the Young Adults Cancers Program, and co-chair of the NCI GI Steering Committee. Dr. Eng, what drew you to this field?
Dr. Eng: What drew me is individuals like yourself. I had a very, very large clinic when I used to work at MD Anderson and I was seeing patients as young as 16. These patients do not have an inherited form of colorectal carcinoma. The majority have sporadic colorectal cancer and, unfortunately, they were showing up in my clinic with very advanced diseases, stages 3 and 4. It’s hard not to be able to appreciate all the hurdles that a young adult has to go through in order to go through their treatment with success and hopefully few toxicities. It’s that communication with the patient and that lives with you as a physician.
Allison: I know many people who are part of the Young Adult Cancers Program and many people who have gone through it. I appreciate everything you’re doing for the young adults who are going through treatment and, unfortunately, the future young adults who will go through treatment. You have to treat the patient as a whole. Things like fertility and body image are so important along with chemotherapy and radiation.
Dr. Eng: Thank you. I want to thank you also for helping promote education and awareness. We don’t know the exact etiology of why this is happening, but in the interim, we can continue to promote awareness so people can be diagnosed earlier.
Allison: Awareness is key.

You don’t need to remove your primary tumor to improve overall survival. In fact, it does not improve overall survival and that’s important to keep in mind.
Dr. Cathy Eng
Latest Treatment Options for Metastatic Colorectal Cancer
Allison: As an advocate, I’ve seen that progress is being made in treating metastatic colorectal cancer, but it’s still challenging. What are the latest treatment options?
Dr. Eng: Oxaliplatin-based and irinotecan-based treatments are still commonly utilized for patients who don’t have a particular molecular marker. We have to recognize that it’s still important to be in contact with the patient because in combination with a fluorouracil-based therapy, those regimens are still the foundation of basic treatment.
Over the past two years, one thing that came out was looking at tumor-sidedness. A patient with a left-sided tumor has a better prognosis overall than a right-sided tumor for a non-inherited form of colorectal cancer. If the patient is RAS wild-type, the data shows that you would probably benefit from anti-EGFR therapy in combination if you’re considering irinotecan- or oxaliplatin-based therapy and that has been shown to improve overall survival. Not everybody loves EGFR therapy because it causes a significant rash, but there are benefits to considering that type of treatment.
A RAS mutation is very common in up to 60% of all colorectal cancer patients. We’re getting so specific that we’re asking what type of RAS mutation you have with regard to KRAS.

Patients ask if they need to have their primary tumor removed because they have metastatic disease. It’s not like breast cancer where you need to have your primary tumor removed to reduce your tumor burden. That doesn’t benefit a colorectal cancer patient with metastatic disease.
A pooled analysis came out in the Journal of Clinical Oncology. Three presentations from countries all across the world indicated that you don’t need to remove your primary tumor to improve overall survival. In fact, it does not improve overall survival and that’s important to keep in mind.
Sometimes we hear from patients that they’re told they have to remove their primary tumor. You shouldn’t have to unless you have uncontrolled bleeding, near obstruction, or a high risk of perforation. We don’t encourage that for all comers in general. For a rectal cancer patient, if you’re having symptoms, we can give short-course radiation therapy to help alleviate some of those symptoms despite having metastatic disease.

HER2-Targeted Therapy Combination
Dr. Eng: Tucatinib is the most recent drug that has been approved, which is an oral tyrosine kinase inhibitor of HER2 that prevents downstream phosphorylation and involves the EGFR pathway. These are for patients with RAS wild-type and most often have left-sided tumors.
The best part about the tucatinib study, which shows the relevance of having a rare mutation, is it got FDA-approved based on a small phase 2 study. It wasn’t a giant phase 3 study and that’s important to keep in mind. It showed that these patients, despite heavily being heavily pre-treated, had a great response rate. Progression-free survival was excellent and the overall survival was close to two years.

HER2 testing is one of the newest things to be considered. We’re very familiar with it in gastric cancer and breast carcinoma, but this is the first time looking at it in metastatic colorectal cancer and making sure that all patients get tested for HER2. It’s extremely important to look at the role of HER2. We have seen that looking at HER2 amplification, which is present in approximately 4% of our patient population, is very, very important.
Looking at HER2 amplification, which is present in approximately 4% of our patient population, is very, very important.
Dr. Cathy Eng
Fam-Trastuzumab Deruxtecan-nxki (ENHERTU)
Dr. Eng: The new drug deruxtecan is an antibody-drug conjugate. It isn’t necessarily new with regard to its presentation but in the sense that it just received tumor-agnostic approval, so it’s approved for colorectal cancer and other tumor types.

It does best in the IHC 3+ patient population, but it has a benefit with regard to progression-free survival and overall survival. What’s unique about deruxtecan is that the original study that resulted in its approval allowed patients who received prior HER2-based therapy. You could have had tucatinib, pertuzumab, or lapatinib, but you can receive deruxtecan as a single agent.

They’re moving the G12C inhibitors in combination with chemotherapy to the front-line setting.
Dr. Cathy Eng
CodeBreaK 300 Clinical Trial
Dr. Eng: CodeBreaK 300 was presented at the 2023 European Society for Medical Oncology Congress. It was a phase 3 clinical trial in a previously treated patient population. They looked at dosing as well.
KRAS G12C inhibitors don’t work by themselves. They were evaluated by themselves with a less than 10% response rate. In combination with anti-EGFR (epidermal growth factor receptor) therapy, the combination resulted in an improved benefit for patients. Although it’s not FDA-approved at this time, if you’ve been previously treated, you can inquire about receiving it in combination with anti-EGFR therapy.

They’re moving the G12C inhibitors in combination with chemotherapy to the front-line setting. In the HER2 setting with newly diagnosed patients, they’re also looking at tucatinib in combination with oxaliplatin-based therapy. The fact that we’ve found molecular alterations that have been positive studies in the refractory setting is exciting. We’re now moving into front-line therapy to see if that is more advantageous for our patient population.
BREAKWATER Trial
Dr. Eng: BREAKWATER is a phase 3 trial for BRAF V600E mutation tumor types, which is about 9% of all of our patients. Encorafenib and cetuximab were approved in the previously treated setting and are now being moved up to the front-line setting in combination with oxaliplatin-based therapy. I’m one of the investigators on that trial and there’s another arm that’s open, which is in combination with irinotecan-based therapy.

I hope that we will continue to move these rare molecular alteration subtypes earlier on in development because we can get greater outcomes. We hope to improve and break the barrier of overall survival, which is currently about 35 to 37 months for the majority of our patients.
For any metastatic patient, the very first thing they need to ask is their molecular sequencing. It’s critical because it helps create a treatment plan.
Dr. Cathy Eng
Trending to More Personalized Treatment
Allison: You talked about the KRAS, HER2, and BRAF mutations, and about treatment being driven by a particular mutation. Why is biomarker testing important? What would you tell a patient if their doctor hasn’t necessarily brought it up?
Dr. Eng: For any metastatic patient, the very first thing they need to ask is their molecular sequencing. It’s critical because it helps create a treatment plan. Previously, we would look at all metastatic colorectal cancer patients as the same and that’s not the case. Having a molecular marker can make a big difference because it can prevent you from receiving unnecessary toxicities and costs.

KRAS- and EGFR-Targeted Therapy
Dr. Eng: The most pivotal one was looking at KRAS and anti-EGFR therapy, such as panitumumab and cetuximab. That class of drugs causes a rash in 80 to 90% of patients and depending on where you live, you’re also at a higher risk for having an allergic hypersensitivity reaction to cetuximab.

Bevacizumab
Dr. Eng: Bevacizumab is an IV drug that’s involved in anti-angiogenic therapy. Anti-angiogenesis or anti-VEGF (vascular endothelial growth factor) therapy means it’s working on the blood supply. Our goal for this class of drugs is to reduce the development of metastatic disease because a tumor needs a blood supply to survive and thrive.

FRESCO-2 Trial
Dr. Eng: When we designed FRESCO-2, it allowed almost all comers. Patients were allowed to have received prior TAS-102 (trifluridine/tipiracil) and that was before the approval with bevacizumab in combination. They also could have received regorafenib, which has been approved for quite a while now. Patients may have been exposed to either or both of the drugs. It was a trial that allowed almost all patients to participate with the median line of four prior lines of therapy.

Fruquintinib was compared to a placebo. It was a 2-to-1 randomization. Some people criticize the fact that there was a placebo arm, but there’s no standard of care for fourth-line or fifth-line therapy, so there was nothing to compare it to. Some people say, “Can’t you go back to 5-FU (fluorouracil)?” But the reality is patients have already progressed through 5-FU, so they’re not going to be receptive to 5-FU.
The primary endpoint was met with an improvement in overall survival as well as progression-free survival.
The FDA approval allows patients to receive oral fruquintinib as a potential option for third-line therapy and beyond.
Dr. Cathy Eng
Fruquintinib (FRUZAQLA) Approved by FDA
Allison: With the recent FDA approval of fruquintinib, how can it help address an unmet need in the metastatic colorectal cancer treatment landscape?
Dr. Eng: It’s a drug that I helped bring to the United States. I was involved in the original trial as the senior investigator. This drug is an oral agent, which specifically blocks receptors 1, 2, and 3 of the VEGF pathway. It was originally approved in China in the third-line setting based on a phase 3 trial called FRESCO that was conducted overseas.
FRESCO-2 is specifically trying to address the practice patterns of what we are doing in the United States, which is continuing bevacizumab for front-line and second-line therapy but also trying to ensure the majority of patients had received anti-VEGF therapy as part of treatment, which was the case in 97-98% of patients who participated in FRESCO-2.
Based on the results of both FRESCO and FRESCO-2, the FDA approval allows patients to receive oral fruquintinib as a potential option for third-line therapy and beyond.

Overall, it was tolerated fairly well. Hypertension is an issue with this class of drugs, but it’s treatable. It got approved in November and we are very pleased to see the FDA approved it. It’s pending the European Medicines Agency’s approval as well as approval in Japan. After that, hopefully, it will be approved internationally so all patients can get access.
At the end of the day, the reality is you need to make sure you have drugs available to the patient. When you ask, “Do you prefer FOLFIRI (folinic acid, fluorouracil, and irinotecan) or FOLFOX (folinic acid, fluorouracil, and oxaliplatin)?” The reality is you’re going to get both regimens. What matters is how well you tolerate the treatment and how you derive the best benefit.
I try not to make it very competitive. I don’t think it benefits anybody. It’s important to recognize that there’s a new drug on the market, it’s available for patients, and it does not require IV therapy.
In a prior study, it was seen that if you have the RAS mutation and receive anti-EGFR therapy, it’s no better than receiving a placebo.
Dr. Cathy Eng
Biomarkers (“Molecular Markers”)
Dr. Eng: There’s a lot of interest with regard to other molecular markers, like a patient’s KRAS status and mutation. KRAS G12C inhibitors are further along in development than G12D. Granted, G12C in colorectal carcinoma is less than 5% of our patient population, but once those patients are identified, they may benefit from therapy.

Importance of Biomarkers
Dr. Eng: The KRAS mutation is in about 30 to 60% of all colorectal cancer patients. In a prior study, it was seen that if you have the RAS mutation and receive anti-EGFR therapy, it’s no better than receiving a placebo. Why would you want to subject your patient to toxicity, a potential allergic reaction, and extreme financial costs if it doesn’t benefit them?

Different Biomarkers
Dr. Eng: KRAS was just the beginning. We are now identifying all these new molecular subgroups, such as HER2, BRAF, and microsatellite instability-high (MSI-H), which is extremely important with regard to the role of immunotherapy. That’s another small patient population, less than 5% of our patients, but that’s where immunotherapy may benefit our MSI-high or mismatch repair protein deficiency (MMR-d) patient population where immunotherapy can have a significant role in potentially curing them.
Tissue molecular sequencing can look at hundreds and hundreds of various molecular alterations, mutations, or amplifications… Information from the blood can be critical in identifying a suitable clinical trial.
Dr. Cathy Eng
Tissue vs. Blood Samples in Biomarker Testing
Dr. Eng: Molecular sequencing can be obtained from tissue and blood. If you’re newly diagnosed, it’s important to send off that tissue as soon as possible before it gets sent off to some warehouse because it can’t sit in the pathology department forever.
Depending on which panel you use, tissue molecular sequencing can look at hundreds and hundreds of various molecular alterations, mutations, or amplifications. Blood sequencing is quicker. You can get it back within less than two weeks, but you’re only looking at 100 different mutations relative to the 500 or so plus potential molecular alterations.
Some places still do it in-house, but the majority of institutions send it out to a third party. Ask for tissue and blood to be sent off because the information from the blood can be critical in identifying a suitable clinical trial for the patient as well. I cannot emphasize enough the importance of participating in clinical trials.

Allison: I’m very, very passionate about access to clinical trials, education for clinical trials, not being scared, and not thinking that a clinical trial means you’re going to die.
Dr. Eng: Every single FDA-approved drug came from a clinical trial.
Allison: Exactly. It’s the point that I make as an advocate all the time.
Choosing Among Different Treatment Options
Allison: Where do you see fruquintinib fitting in with the current therapies available for metastatic colorectal cancer? Is there a potential for it to be used as a maintenance therapy?
Dr. Eng: My understanding is it’s being investigated as a maintenance therapy. It makes a lot of sense since the majority in the United States use capecitabine, bevacizumab, or 5-FU with bevacizumab for maintenance therapy, so it would make sense to consider this as a potential maintenance therapy.
When thinking about it relative to other agents, such as regorafenib and TAS-102 plus bevacizumab, I talk to the patient. I utilize TAS-102 plus bevacizumab quite a bit. However, some patients are tired of coming in every two weeks for bevacizumab. They want a break and that’s very reasonable.

With TAS-102, they’re at risk for myelosuppression, so their white blood cell count can drop. That’s a known side effect of that class of drugs, so the patient has to be compliant and not take medications incorrectly. You don’t want a neutropenic patient walking around at risk for other infections.
I don’t offer TAS-102 to certain patients. I had an elderly patient whose platelet count was never above 100,000. He had been on a lot of therapies and came to me for a second opinion. I would be hesitant to give him TAS-102 because I know that his platelet count is going to be a problem, so I chose fruquintinib for him first. Once again, I don’t try to choose one versus the other. I talk to the patient about the potential side effects and see what they’re willing to endure.
Allison: It’s all about shared decision-making. You know about what’s going on and having that conversation with your physician is very, very important.

Emerging Clinical Trials for Metastatic Colorectal Cancer Patients
Dr. Eng: For the MSI-high patient population, there should be some updates soon regarding NIVO+IPI (nivolumab and ipilimumab). They presented their data at the 2024 ASCO GI (American Society of Clinical Oncology Gastrointestinal Cancers Symposium) looking at NIVO+IPI in combination versus chemotherapy and that was superior, but it was a very rapid abstract presentation, so we don’t have further details. I think there’s going to be an update at the 2024 ASCO, so I look forward to that. Are two immunotherapies better than one for this patient population? What about toxicities? It will be interesting to see.
The CodeBreaK 301 trial is looking at the role of sotorasib, a first-generation KRAS G12C inhibitor, in the upfront setting. Sometimes the next generation may be even better, so I’m looking forward to those trials as well.
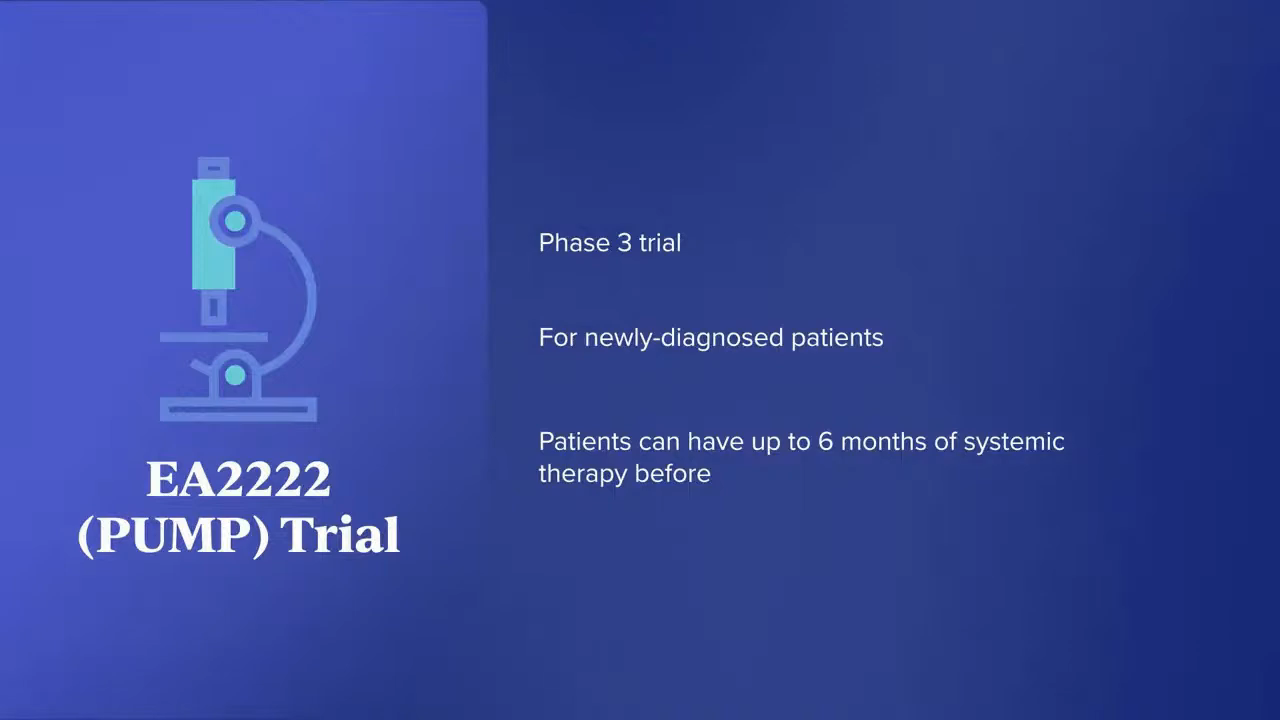
EA2222 (PUMP) Trial
Dr. Eng: The PUMP trial is a new NCI-sponsored study specific for newly diagnosed patients. They will get randomized to having the PUMP versus continuing chemotherapy.
This is an important trial that will answer that question. We know all the data from Memorial Sloan Kettering, but can we validate and replicate that data outside of that institution, especially in an earlier line setting?

ERASur Trial
Dr. Eng: The ERASur trial is another NCI-sponsored study that looks at the role of stereotactic radiation therapy, ablation, or some type of liver-directed intervention to improve overall survival.
MOUNTAINEER-03 Trial
Dr. Eng: The MOUNTAINEER-03 trial is looking at tucatinib with trastuzumab in the front-line setting.
We cannot make breakthroughs until we get trials completed. It’s very, very important to keep in mind that not all trials involve a placebo.
Dr. Cathy Eng
Importance of Participating in Clinical Trials
Allison: We touched on the importance of clinical trials, but do you have any message about the importance of participating in clinical trials?
Dr. Eng: Focusing on metastatic disease, I encourage all patients to consider participating in a clinical trial. Currently, enrollment across the United States is about 7% for non-NCI cancer centers and up to 20% for NCI-designated cancer centers. We cannot make breakthroughs until we get trials completed. It’s very, very important to keep in mind that not all trials involve a placebo.
All patients should consider getting a second opinion if they haven’t been offered a clinical trial. There are some selective settings where there is no clinical trial available, but you should have that discussion with your provider on your first or second meeting.
It doesn’t take that long to get a second opinion. You want to look at all your options and feel informed. That’s extremely important as a patient and the best way to optimize your care. As a medical oncologist, it’s very, very distressing when I meet a patient who received one cycle of chemotherapy before they walked into my office and find out they would have been a perfect candidate for a clinical trial but now they’re automatically disqualified.
Allison: Knowledge is power. There are so many amazing trials and the first step is educating yourself on clinical trials. There are a lot of advocacy groups and organizations that are trying to educate patients on what a trial is and having that conversation with their doctors. It’s so important because the last thing you want to do is disqualify yourself for a trial because you did something here versus going there.
Because of my age, I got three different opinions before I decided on where I was going to get treated. For the younger people especially, talk to as many people as you can and educate yourself as much as you possibly can. This program is an amazing start for people to better understand that there are options.
Get a second or a third opinion. I’m not telling you to delay your treatment by months, but give yourself 2 to 3 weeks to make an informed decision by getting another opinion.
Dr. Cathy Eng
Final Takeaways
Allison: What message do you have for metastatic colorectal cancer patients? I always tell them that their lives aren’t over. There are treatment options available. I know quite a few people who were stage 4 who are now in remission.
Dr. Eng: I hear time and time again that they’ve been given a poor prognosis of 6 to 8 months to live; that’s very discouraging and not the right approach. We have so many new options available. Science has advanced so much. If you hear that kind of negative attitude at your first meeting with your physician, you need to go get a second or a third opinion. I’m not telling you to delay your treatment by months but give yourself 2 to 3 weeks to make an informed decision by getting another opinion.
Allison: I agree. The relationship you have with your care team is important. It can spell the difference between a lot of different things that can happen along a journey.
Dr. Eng: It’s also important for people to keep in mind that there’s always hope.
Allison: I was originally diagnosed with stage 3 and they said it was about to break through my colon wall. There’s so much going on and so much information you’re getting when you’re first diagnosed. Talking to someone else who’s gone through it gave me hope. It differs with every stage. I know people who are 20-year stage 4 colorectal cancer survivors. That’s the hope that someone needs.
Dr. Eng: Your medical oncologist is key. Your radiation oncologist and surgical oncologist are always there, but the captain of your ship, besides your primary care doctor, is your medical oncologist.
Allison: You are an amazing captain of the ship. I know this from hearing from many people and knowing you for as long as I have. Thank you so much for your time and for sharing invaluable information.
Stephanie: Thank you so much, Dr. Eng, for what you do in terms of research, which is critical to advancing care, and the way you’re thinking about patients. Thank you also to Allison for the work you do in advocacy and leading this program, which wouldn’t be the same without the patient perspective.
Thank you to our promotional partner the Colon Cancer Coalition, which was founded by someone who lost her sister to stage 4 colorectal cancer. The Colon Cancer Coalition is celebrating its 20th Get Your Rear in Gear fundraiser in the Twin Cities in Minnesota with other rides and fundraisers happening across the country as well. These fundraisers highlight the need to screen and raise awareness of colorectal cancer. The organization gives more than $1 million every year to local community programs to help fill that need.
We hope that you walk away with a better understanding of what’s out there and something to talk to your own healthcare team about. We hope to see you at a future program.
If you read and/or watched our program, we encourage you to take our program survey to help us improve future programs and answer your questions.