FDA Approves New Treatment Options for Relapsed/Refractory Multiple Myeloma Patients
In a significant stride forward for the field of multiple myeloma treatment, the U.S. Food and Drug Administration (FDA) has recently granted accelerated approvals for two innovative therapies, elranatamab-bcmm (Elrexfio) and talquetamab-tgvs (TALVEY).
These approvals mark critical advancements in addressing the complex challenges faced by adults with relapsed or refractory multiple myeloma, a condition characterized by resistance to previous therapies. Elranatamab and talquetamab offer renewed hope to patients who have exhausted prior treatment options, providing novel avenues to combat the disease’s progression and improve clinical outcomes.
Let’s delve into the details of these groundbreaking treatments and explore how they are reshaping the landscape of multiple myeloma care.
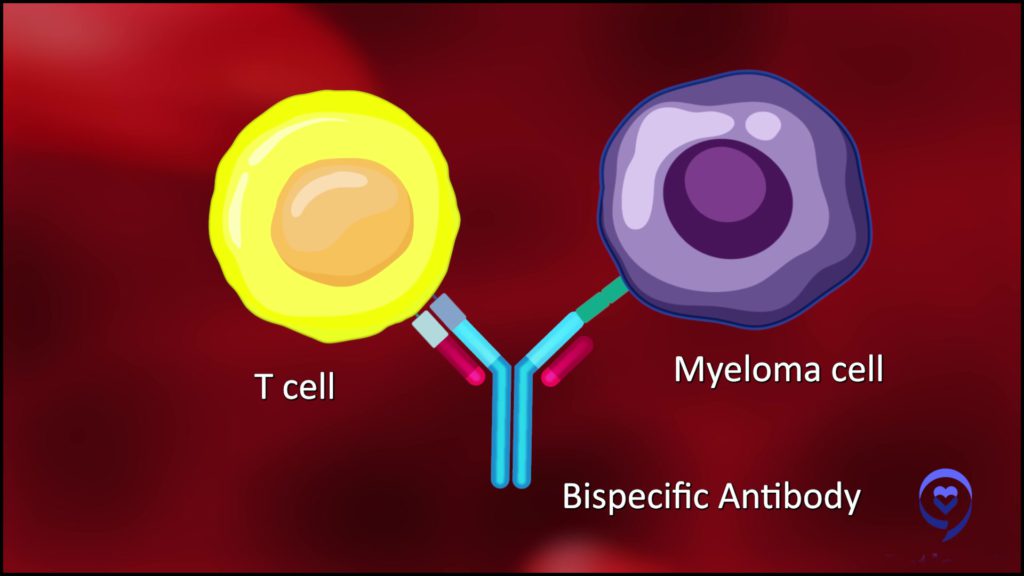
How bispecific antibodies work

“A bispecific antibody is a molecule that’s got two arms. One arm grabs a T-cell and the other arm grabs a multiple myeloma cell by recognizing a target on the multiple myeloma cell, just like the CAR T-cell does.
This bispecific can grab a myeloma cell with one arm, grab a T-cell with another arm, bring them together, and force that T-cell to recognize the multiple myeloma cell.”
Dr. Alfred Garfall | Explore Expert Q&A on Bispecific Antibodies
Talquetamab-tgvs FDA Approval
Another treatment option has been added for multiple myeloma patients after the U.S. Food and Drug Administration (FDA) granted accelerated approval to talquetamab-tgvs (TALVEY) for adults with relapsed or refractory multiple myeloma (RRMM). To get access, these patients must have already gotten four lines of therapy, including an anti-CD38 monoclonal antibody, immunomodulatory agent (“IMiDs”), and proteasome inhibitor.

“Recent approvals have focused on triple-class refractory patients (refractory to IMId, PI, Mab), by using BCMA-based therapies ( ide-cel, cilta-cel, teclistamab). Talquetamab looks beyond this to patients who have progressed beyond BCMA-based therapies and offers new hope.”
Dr. Joshua Richter
The approval comes following a phase 1/2, open-label, multicenter clinical trial dubbed MonumenTAL-1, a study that included 187 RRMM patients through at least four lines of therapy as described above. The drug manufacturer, Janssen Pharmaceutical, made the announcement in this Aug. 10 press release. It’s important to note that the approval hinges on “continued approval for this indication is contingent upon verification and description of clinical benefit in confirmatory trial(s).”
Talquetamab, or TALVEY, is a bispecific antibody approved for myeloma patients, targeting the GPRC5D receptor, with a reported 70-percent of durable responses that include a patient population that had already undergone a different bispecific antibody or CAR T-cell therapy.
“Recent approvals have focused on triple-class refractory patients (refractory to IMId, PI, Mab), by using BCMA-based therapies ( ide-cel, cilta-cel, teclistamab). Talquetamab looks beyond this to patients who have progressed beyond BCMA-based therapies and offers new hope,” said Joshua Richter, M.D., Director of Multiple Myeloma at the Blavatnik Family-Chelsea Medical Center at Mount Sinai in New York.
Myeloma advocacy organizations seem aligned in offering another treatment option to people who’ve already been through so much treatment and had more limited options.
“Although options for the treatment of multiple myeloma have expanded significantly in recent years, the disease remains incurable, and therefore, patients are in need of new treatment options,” said Michael Andreini, President and Chief Executive Officer, of Multiple Myeloma Research Foundation in the Janssen release. “[The] approval of talquetamab provides patients with a new treatment approach for relapsed or refractory disease that is a welcome addition to the myeloma community.”
Elranatamab-bcmm FDA Approval
For the second time in a month, the FDA has granted accelerated approval to a bispecific antibody for refractory multiple myeloma patients. Elranatamab-bcmm (Elrexfio) is a bispecific B-cell maturation antigen (BCMA)-directed CD3 T-cell engager.
The approval is aimed at adults facing relapsed or refractory multiple myeloma, who have undergone a minimum of four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
According to the FDA press release, the accelerated approval for elranatamab was grounded in findings from the phase 2 MagnetisMM-3 clinical trial. This multicenter, open-label, single-arm study involved individuals battling relapsed or refractory multiple myeloma, who had undergone previous treatments, including a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 antibody.
“Most multiple myeloma patients will experience relapse or resistance of their disease to treatment, often facing increased symptom burden and lowering their chance of surviving longer with each attempted line of therapy,” said Ajay Nooka, MD, MPH, director of the multiple myeloma program at Winship Cancer Institute of Emory University in Atlanta, in a press release from drug manufacturer Pfizer. “By offering durable clinical response with an established safety profile and the convenience of subcutaneous administration, [elranatamab] provides a much-needed new option for heavily pre-treated multiple myeloma patients who are struggling with relapsed myeloma.”
»MORE: The Future of Multiple Myeloma Treatment: Expert Q&A on Bispecific Antibodies
Multiple Myeloma Stories
Clay D., Relapsed/Refractory Multiple Myeloma
Symptoms: Persistent kidney issues, nausea
Treatments: Chemotherapy (CyBorD, KRd, VDPace), radiation, stem cell transplant (autologous & allogeneic), targeted therapy (daratumumab), immunotherapy (elotuzumab)
...
Melissa V., Multiple Myeloma, Stage 3
Symptom: Frequent infections
Treatments: IVF treatment & chemotherapy (RVD) for 7 rounds
...
Elise D., Refractory Multiple Myeloma
Symptoms: Lower back pain, fractured sacrum
Treatments: CyBorD, Clinical trial of Xpovio (selinexor)+ Kyprolis (carfilzomib) + dexamethasone
...
Marti P., Multiple Myeloma, Stage 3
Symptoms: Dizziness, confusion, fatigue, vomiting, hives
Treatments: Chemotherapy (bortezomib & velcade), daratumumab/Darzalex, lenalidomide, revlimid, & stem cell transplant
...
Ray H., Multiple Myeloma, Stage 3
Symptoms: Hemorrhoids, low red blood cell count
Treatments: Immunotherapy, chemotherapy, stem cell transplant
...





