Follicular Lymphoma: Latest Advances in Precision Medicine and Emerging Therapies
Dr. Peter Martin, a leading lymphoma expert at Weill Cornell Medicine, and Laurie Adami, a follicular lymphoma patient discuss the latest advancements in follicular lymphoma treatment. This conversation talks about precision medicine and emerging therapies, addressing how to manage side effects and improve quality of life, and is designed to empower patients with practical knowledge and support as they navigate their diagnosis.
Understand current and emerging options, including targeted therapies and bispecific antibodies, and how they address treatment challenges. Gain actionable strategies to manage side effects and improve quality of life. Explore how precision medicine tailors treatment plans to individual needs, including chemo-free options. Get answers to common and critical questions about follicular lymphoma. Be part of a conversation that brings the patient experience front and center to inspire hope and informed decision-making.

Thank you to The Leukemia & Lymphoma Society for their partnership. The Leukemia & Lymphoma Society is here for you with information about clinical trials, resources, and support.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make treatment decisions.
- Introduction
- What is Follicular Lymphoma?
- How is Follicular Lymphoma Diagnosed?
- Symptoms of Follicular Lymphoma
- Traditional Treatments for Follicular Lymphoma
- Bispecific Antibodies for Treatment of Relapsed/Refractory Follicular Lymphoma
- Experience with CAR T-cell Therapy
- FDA-Approved Bispecific Antibodies for Follicular Lymphoma
- Why are Bispecific Antibodies Administered in the Clinic or Hospital?
- Side Effects of Bispecific Antibodies
- Personal Experience with Side Effects
- Precision Medicine as an Approach to Follicular Lymphoma
- Long-Term Implications of Chemo-Free Treatments for Follicular Lymphoma
- Clinical Research and Follicular Lymphoma
- Fertility Preservation and Follicular Lymphoma
- Bridging the Gap Between Academic Research Centers and Community Hospitals
- Key Takeaways from ASH
- Conclusion
Introduction
Tiffany Drummond: I’m a patient advocate with over 20 years of experience in cancer research. My journey as a care partner began when my mother was diagnosed with endometrial cancer in 2014. I quickly realized the challenges of finding resources, support, and shared experiences, and now I am committed to helping others avoid similar difficulties, no matter the condition.
At The Patient Story, we create programs to help you figure out what comes next. Think of us as your go-to guide for navigating not only the cancer journey but your overall health journey. From diagnosis to treatment, we have you covered with real-life patient stories and educational programs with subject matter experts and inspirational patient advocates and guests. I genuinely am your personal cheerleader, here to help you and your loved ones best communicate with your healthcare team as you go from diagnosis through treatment and survivorship.

The Patient Story retains full editorial control over all content as always. We also thank all of our promotional partners for their support. It is because of you our programming reaches the audience who needs it. I hope you’ll find this program helpful, but please keep in mind that the information provided is not a substitute for medical advice.
I had access to great cancer centers. I went to four different big cancer centers and that’s where I was able to join clinical trials.
Laurie Adami

Laurie Adami, Follicular Lymphoma Patient Advocate
Tiffany: I have the pleasure of interviewing Dr. Peter Martin and it so happens that we have much more in common than you might think. I will also be speaking with a follicular lymphoma patient, Laurie Adami. It’s so important to get a patient’s perspective and I’m sure her experience will resonate with you and your loved ones. Let’s learn about Laurie’s journey before deep diving into the latest treatment options.
Laurie Adami: I was diagnosed in 2006. My son was in kindergarten. There was one treatment I did right away. We thought I was in remission, so I merrily went back to work, which entailed traveling internationally. Three months later, my cancer was back on the first follow-up scan. That prompted 12 years of treatment. From 2006 to 2018, I was in continuous treatment and underwent seven different lines of therapy, including three clinical trials.
The first six treatments didn’t work, but thankfully, the seventh line of treatment did. I live in Los Angeles, so I had access to great cancer centers. I went to four different big cancer centers and that’s where I was able to join clinical trials.

Dr. Peter Martin, Hematologist-Oncologist
Tiffany: Dr. Peter Martin serves as the professor of medicine and chief of the lymphoma program at Weill Cornell Medicine. He is a hematologist-oncologist who specializes in caring for patients with lymphoma at NewYork-Presbyterian Hospital. His research focus, which is very dear to my heart, is on clinical investigation of new and promising therapies. Dr. Martin, how are you doing today?
Dr. Peter Martin: It’s great to see you again, Tiffany. I don’t know if everybody else knows this, but we worked together long ago.
Tiffany: You could say how long ago. It was about 15 years ago. I used to call him Peter, that’s how far we’ve come. I’m glad to see that we both have stayed the course, which is fighting cancer and finding a cure. I know that you made great strides, so thank you for being here.

[Follicular lymphoma] typically grows slowly over months, years, or decades, and that’s why we call it indolent, which means lazy.
Dr. Peter Martin
What is Follicular Lymphoma?
Tiffany: I’m a patient advocate for many types of cancer, but for those who aren’t as familiar, can you break down follicular lymphoma? We know that it’s considered an indolent lymphoma. Can you walk us through that characteristic? What makes follicular lymphoma distinct from more common types of non-Hodgkin lymphoma, such as DLBCL or diffuse large B-cell lymphoma?
Dr. Martin: Follicular lymphoma is pretty common from the lymphoma perspective but not common from a cancer perspective. In the United States, about 20,000 people every year will be diagnosed with follicular lymphoma. Depending on your perspective, there are up to 130 different kinds of lymphoma, which is hard to keep track of. Each subtype is a little bit different biologically in how they’re defined and how they behave clinically.
Follicular lymphoma is based on the way it looks under the microscope. They look like little follicles in the lymph node. It typically grows slowly over months, years, or decades, and that’s why we call it indolent, which means lazy. I like the word lazy because it’s a lymphoma that can’t be bothered to cause problems.
Once diagnosed, we’ll often talk about the goal of treatment, which is to help somebody live a life that’s as close to the life they would live without lymphoma. It hangs around for a long time and we keep managing it and kicking the can down the road.
How is Follicular Lymphoma Diagnosed?
Tiffany: If someone is living with follicular lymphoma for years or even decades, how is it diagnosed? Do they come in for some other type of symptom that usually makes them get referred to a specialist? How does that work for a patient who doesn’t even know that they have it?
Dr. Martin: It can vary a little bit. Ultimately, to diagnose follicular lymphoma, you have to look at it under the microscope. The word lymphoma means tumors of the lymph nodes, so most of the time when we meet somebody with follicular lymphoma, they’ll have an enlarged lymph node. Typically, it’s a painless, enlarged lymph node in the neck, armpit, or groin.
It might be picked up accidentally while getting tested for other reasons, which is pretty common because follicular lymphoma is often asymptomatic. Although it’s called lymphoma, sometimes it’s not in a lymph node. It might be present in the bone marrow or some other organ, like the gastrointestinal tract or the skin. Ultimately, though, somebody has to look at it under the microscope and that’s how we make the diagnosis.
All of my doctors dismissed me. I would specifically tell them, ‘I’m very worried I have lymphoma,’ and they would say, ‘Your bloodwork is all normal, so you don’t have cancer.’
Laurie Adami
Symptoms of Follicular Lymphoma
Tiffany: Laurie, did you experience any symptoms before your diagnosis or was it something you noticed but didn’t read into it enough to get it checked right away?
Laurie: When my son was three years old, I started to get frequent sinus infections and couldn’t get rid of them. I also developed a dry eye. I was a long-time contact lens wearer and suddenly, I couldn’t wear my right contact lens. I was very tired. I had a lymph node on my neck that was concerning me and I felt something in my abdomen.
All of my doctors dismissed me. I would specifically tell them, “I’m very worried I have lymphoma,” and they would say, “Your bloodwork is all normal, so you don’t have cancer.” I told my husband that they wouldn’t listen to me and he didn’t believe me, so I took him to my appointments and he couldn’t believe how I was dismissed. He was mortified. This went on for three years.
I wanted to believe these doctors. I wanted to believe that allergies were causing these sinus infections. I was also at the age where I could be starting to get perimenopausal symptoms, so my symptoms were attributed to my hormones. It was aggravating.
I kept feeling worse and worse. The exhaustion was incredible. One of the doctors I saw said, “Laurie, you’re president of a software company. You’re traveling internationally. You’re traveling a week a month. You’re running a household. You have a young boy. I’m exhausted just listening to what you do.”
Finally, someone I knew referred me to a diagnostician who took me seriously. I went in to see him and explained everything. I said, “People think this node is from allergies.” He said, “Did you get tested for allergies?” I said, “Yeah, and there was nothing.” He said, “Okay, that doesn’t make sense then.”
I explained the possibility of a hernia and he said, “It could be, but Laurie, we don’t guess. We have CT machines and I’m going to send you to a hernia specialist. We’re going to do imaging.” That week, they imaged me and it was scary because I was supposed to go in for a simple imaging. They expected to find a hernia and I wasn’t supposed to have contrast. Suddenly, this lady brought in the bottles of contrast because they had to get a better image. My heart began to sink as I was sitting there.
Two days later, on Good Friday of 2006, the doctor’s office called and said I needed to come in. My mother and brother were in town for the Easter weekend, but they didn’t tell me to bring anyone, so I went by myself and they told me I had either lymphoma or a mesenchymal tumor. The imaging also detected lesions on my lungs, so he said, “You may also have lung cancer.” That’s when I found out that I had some type of cancer.
I knew a little bit about lymphoma because when I had this node and I put in my symptoms online, lymphoma kept coming up. But 90% of lymphoma patients are diagnosed at stage 4 because most patients don’t have symptoms. I did, but I didn’t have anybody listening to me, so they dismissed my concerns.
As it turned out, it was good that they didn’t listen to me because the only treatment that existed at the time was a monoclonal antibody, multi-chemo, prednisone treatment. If I had it earlier, it wouldn’t have worked and I would have had nothing else.
I had autologous stem cell transplant as an option, but it was not a good option, especially if you relapse after the first line of chemo and monoclonal antibody treatment. In a way, it ended up being a blessing because, by the time they were pushing me to do the transplant, I managed to find a trial of a histone deacetylase (HDAC) inhibitor, which is what I did as my second line of therapy.
Dr. Martin: Oftentimes, somebody will say, “I’ve had this lump for five years and finally my friend told me that I should have it looked at.” That’s a testament to how it behaves. It hangs around for a long time, so people often get accustomed to it being there before they decide to bring it to somebody’s attention.

Traditional Treatments for Follicular Lymphoma
Tiffany: Before we get into the novel approaches, can you walk me through the traditional treatments for follicular lymphoma? What factors determine a more or less aggressive approach in your practice?
Dr. Martin: The job of a hematologist-oncologist is to learn as much as we can about the lymphoma and that’s changing all the time as we get more sophisticated. We recognize that this lymphoma isn’t happening in a petri dish in a lab somewhere; it’s happening in a real live person, so we have to learn as much as we can about that person. That includes not only their medical issues but also all of the things that are important to them. What are their values? What is their support network like? There are 8 billion of us on the planet and we’re all different, so there’s even more heterogeneity among people than there is amongst the 130 different lymphomas.
We bring all of that information together and come up with a plan that makes the most sense for that person. Treatments are constantly evolving and we have new treatments available today that we didn’t have in the past. In general, the goal of treatment is to try to give somebody the life that’s the closest to the life that they would have if they didn’t have lymphoma.
We have to learn as much as we can about that person. That includes not only their medical issues but also all of the things that are important to them.
Dr. Peter Martin
Approaches can vary. In some cases, you say, “Look, this is not causing you any problems. It’s not going to cause problems hopefully for a long time — on average, multiple years — and so we watch it and it sits there.” That can be a little bit counterintuitive. In the Western world, you want to catch and deal with cancers early. That’s certainly the case for breast cancer, lung cancer, or colon cancer, so the initial discussion around follicular lymphoma can be a little bit awkward sometimes. You say, “Oh, great. No problem. Let’s do nothing about it.” But it’s a proven strategy to help people live a good quality of life without dealing with any of the side effects of treatment.
On the other end of the extreme, we might propose using more aggressive therapies, including chemotherapy if we need to shrink something quickly and help them feel better. There are options in the middle where we use immunotherapies that may shrink the tumor, may help somebody to feel better, and may prolong the time between that and other kinds of therapies by months or years. There are vast options and more new treatments are being approved.

Bispecific Antibodies for Treatment of Relapsed/Refractory Follicular Lymphoma
Tiffany: Let’s talk about some of the data that came out of the 66th American Society of Hematology (ASH) annual meeting. Bispecific antibodies are changing the way that we look at and treat cancer, especially when it comes to immunotherapy. What benefit may they contribute to our relapsed/refractory follicular lymphoma patients?
Dr. Martin: Bispecific antibodies are a type of monoclonal antibodies. Antibodies are proteins that our immune system makes to fight against bacteria. A few decades ago, clever people figured out how to make antibodies that would fight not against infections but against cancer. That moved quickly from the lab to people and, for the past 25 years, has revolutionized the way many cancers are managed. It started with lymphoma. We’ve been leading the way for a long time.
Bispecific antibodies are a natural evolution of trying to come up with ways to make this kind of immunotherapy work better. They’re very cleverly engineered. They bind to tumor cells the same way an antibody would bind to a virus or bacteria, but in addition, they also bind to other parts of our immune system called T cells and they activate them. When those T cells are activated, they secrete chemicals called granzymes and perforins that poke holes in cancer cells and cause them to die. This is a clever way of using our immune system to kill cancer cells and it does it remarkably effectively.
The vast majority of people will have not only responses to this treatment, meaning the tumor shrinks, but in many, if not most, cases, the tumor will disappear on a CT scan. It might be completely gone — we’ll find out as the years go by — but it disappears on a CT scan in a lot of people.
Bispecific antibodies are attractive in that it’s a non-chemotherapy approach and it’s a proven form of immunotherapy. It’s an evolution of the kinds of immunotherapies that we’ve been using in the past and it’s more effective.
The other thing that’s nice about it is that it’s off the shelf, so you order it from a pharmacy. It doesn’t have to be engineered specifically for each patient the way something called chimeric antigen receptor T cells or CAR T-cells have to be.
I did a monthly infusion of a third line monoclonal antibody… but as soon as I stopped, it came back, so it was a race against time.
Laurie Adami
Experience with CAR T-cell Therapy
Tiffany: Laurie, I believe you’re familiar with CAR T-cell therapy. How was that experience and where are you currently in your cancer journey? Are you also familiar with bispecific antibodies? I would love to get your perspective on this immunotherapy versus CAR T-cell therapy.
Laurie: I heard about CAR T-cell therapy six years before I could get it. I heard about it in 2012 when I attended an LLS event where they showed Emily Whitehead’s film. I went to my college that week and asked about it because I didn’t know about CAR T-cell therapy. He said, “They’re not trying it for follicular lymphoma. They’re doing it for more aggressive tumors. We have to wait. You’re on a PI3 kinase inhibitor. We’re going to ride this horse.” That took me through 2016 when the cancer finally outsmarted that pill.
A new monoclonal antibody had been approved. It was a nine-month course, so I did a monthly infusion of a third-line monoclonal antibody, obinutuzumab. It immediately started shrinking my tumors again, but as soon as I stopped, it came back, so it was a race against time.
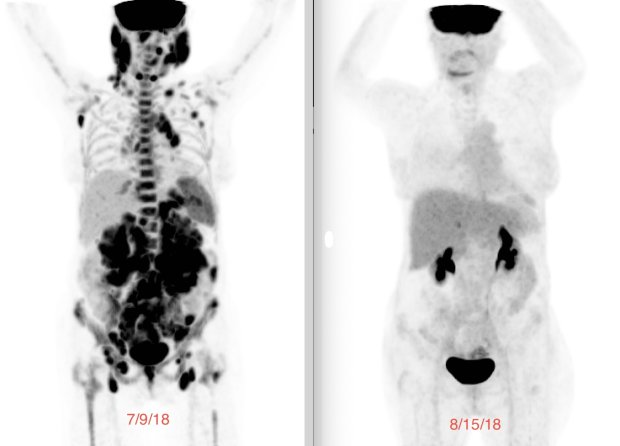
In 2018, my tumors were huge. While I was out hiking in April, my oncologist called and said, “We finally got the trial for follicular lymphoma. It’s going to open at UCLA. We’re going to have five patients enrolled in the first cohort and you will be patient number one.”
When you’re in a clinical trial, you have to review the paperwork to sign. It discloses all the side effects of patients in the phase 1 study. This was a phase 2 study, so it wasn’t completely bleeding edge. Then they have to do biopsies and imaging. They had to make sure I didn’t have anything in my brain. They weren’t allowing patients with central nervous system involvement to get CAR T-cell therapy because they didn’t know how it would work and what it would do. Now they know, so they do it for people with involvement in the central nervous system.
It was amazing because, within days, the tumors were shrinking.
Laurie Adami
I had a sinus infection again because my cancer was coming back. My oncologist said, “You can’t get CAR T-cell therapy with an active infection,” so I went to my ear, nose, and throat specialist. I explained, “I need to get this treatment, but I can’t do it with an active infection.” He called his scheduler and said, “Clear the schedule tomorrow. I have an urgent patient.” They operated on me the following day and it ended up being a major surgery with general anesthesia because there were so many blockages everywhere. He cleaned me out and got rid of the sinus infection so I was good to go.
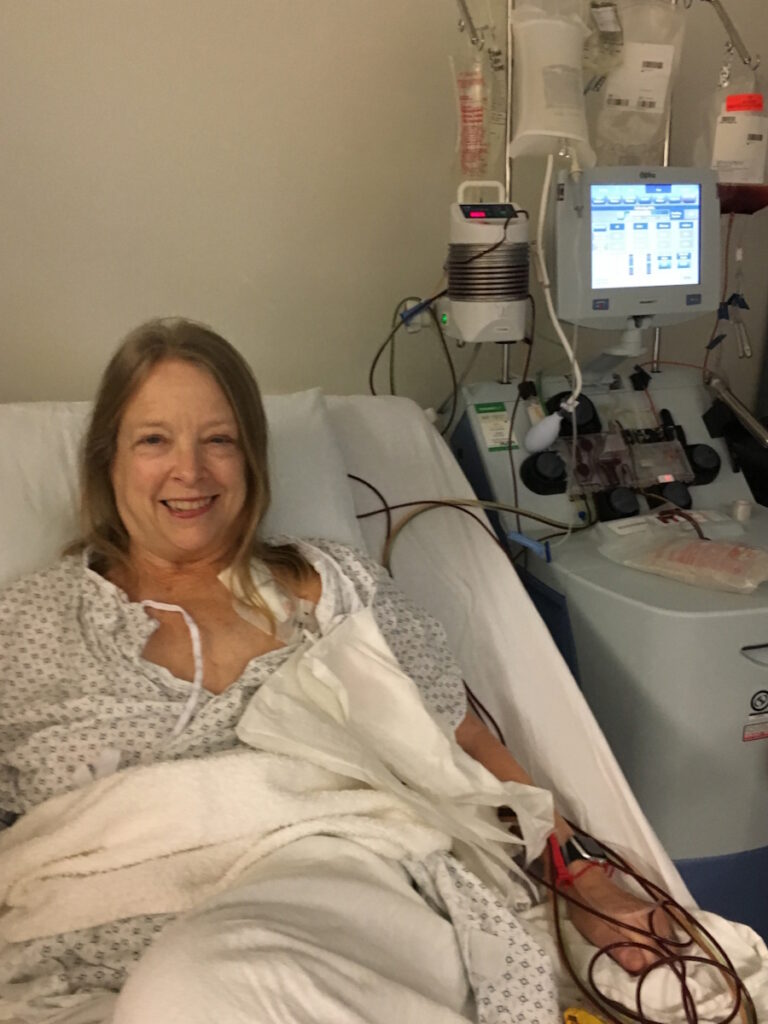
About a month before you get your cells back, they do apheresis. They harvest your T cells. It’s a very easy process that takes half a day outpatient. The courier ships your bag to the CAR T company, which happens to be down the highway in LA. I remember the courier came to pick it up in the apheresis center and I said, “Do not let my cells fall out on the freeway. Please make sure the van door is tightly sealed.” He said, “Don’t worry. We’ll get it there, Laurie. No problem.”
CAR T-cell therapy is about an 18-day process. They shaved off a couple of days and shortened it even more, so the patient didn’t have to wait that long. They take your cells, put the target on them, and grow them in the lab. They harvested about a million cells from me and after they became CAR T cells, there were a billion cells that would get infused.
Before you get your CAR T cells, you go through lymphodepleting chemotherapy, which is chemo light compared to the 18 cycles that I had. It makes room in your bloodstream for you to get your CAR T cells back and gives them room to expand. After three days of lymphodepleting, you get the cells back on day zero, your CAR T birthday. It was amazing because, within days, the tumors were shrinking.

FDA-Approved Bispecific Antibodies for Follicular Lymphoma
Tiffany: Are bispecific antibodies available to patients outside of a clinical trial? Is there anything we can use now straight from a pharmacy without having to go to our investigational one?
Dr. Martin: Two bispecific antibodies are approved for follicular lymphoma: mosunetuzumab and epcoritamab. There probably will be a third one very soon called odronextamab. They’re all pretty similar in terms of how they work and the proteins they target on the surface of the B cells.
There are more coming that we will continue to see in lymphoma and across all cancers. They’re all administered in the clinic or the hospital, so these are not pills that you take at home the way a lot of cancer therapy has transitioned. They’re administered either through an intravenous injection or a subcutaneous injection.

Why are Bispecific Antibodies Administered in the Clinic or Hospital?
Tiffany: Is there a reason for that? Is it because we want to watch for any side effects immediately or is it because of the toxicity and potency of the drug itself?
Dr. Martin: A little bit of both. These are big proteins. They have to be administered by needle because they have to bypass the gastrointestinal tract.
There are some side effects. The side effects that somebody might experience during the infusion are minimal. That said, somewhere in the range of hours to even a couple of days after the treatment, there can be cytokine release syndrome, which happens in up to a third of patients getting these antibodies.
Cytokine release syndrome sounds complicated, but… it’s very manageable.
Dr. Peter Martin
Cytokine release syndrome sounds complicated, but it’s what I described. T cells secrete these chemicals, the same chemicals you experience when you have an infection, including fever, feeling rundown, muscle aches, and what you feel when you have the flu. But in some cases, it can be a little bit more severe. Not very common, but it can happen. We’re often able to manage it with acetaminophen. Sometimes we have to use steroids, like dexamethasone, and rarely do we even have to use other medications.
Because of that, there’s very careful preparation at the facility level, the physician and nursing level, and the patient and caregiver level. It’s all about preparation, helping people to know what to recognize and what to do if something like that happens. It’s very manageable, but it’s a little bit more complicated than taking a pill.

Side Effects of Bispecific Antibodies
Tiffany: Patients are concerned about side effects in general and we know when it comes to cancer, a lot of these drugs are toxic, even though we’ve drastically reduced that over time. In your experience, how do bispecific antibodies differ from traditional chemotherapy and CAR T-cell therapy in terms of side effects? Are they more severe, less severe, or not as long? And does the impact on quality of life determine which avenue they want to take for their treatment?
Dr. Martin: It’s not such a straightforward question to answer because everybody’s different and everybody’s situation is different. Where better-tolerated treatments might be appropriate for one person, more aggressive treatments with more side effects might be appropriate for another person. In most cases, we have options among all of these and oftentimes, there are no wrong or right answers. We try to pick one, but in some cases, we’re driven to say this is the right answer. It’s the job of the whole team — the patient, the caregiver, the physician — to try to pick the right treatment.
Where better-tolerated treatments might be appropriate for one person, more aggressive treatments with more side effects might be appropriate for another.
Dr. Peter Martin
Bispecific antibodies are straightforward in that they can be administered in an outpatient setting or at least partly in an outpatient setting. They don’t cause a lot of the side effects of traditional chemotherapy, like hair loss and nausea, which aren’t major issues with a lot of chemotherapy that we use, but we’re understandably scared of them. Hair loss is a particularly interesting one in that it’s a signal to the rest of the world about something you’re undergoing privately. It tells them publicly that something’s going on, so I understand why that’s not attractive.
Personal Experience with Side Effects
Tiffany: It’s important to get a patient’s perspective regarding side effects. Laurie, can you give us an overview of your experience with side effects? Were there any that you found particularly taxing on you or that affected your quality of life? Were you able to manage your symptoms relatively well?
Laurie: It was a mixed bag. With the first chemo, I lost my hair and got mouth sores. With the second treatment, which was a targeted therapy, I was very, very fatigued. I also lost my hair. They told me that it wasn’t from the trial drug, but when I dug into it, I saw a very small percentage of patients lost their hair.
White counts typically would get depleted, which made me prone to getting infection… so I was a real early adapter of mask-wearing.
Laurie Adami
My fourth treatment was radioimmunotherapy and I had very, very low counts for a long time. My platelets dropped. I never had to get an infusion of platelets, but I had to go in every day to get it checked. I had to be very careful not to fall because I had no clotting ability with low platelets.
White counts typically would get depleted, which made me prone to infections, so I had to be careful. When I went back to work after my first chemo treatment in 2006 and had to start traveling again, I asked my oncologist, “Is it okay if I travel to New York, Boston, London, etc.?” She said, “Yes, but you have to wear a mask,” so I was a real early adapter of mask-wearing.

Precision Medicine as an Approach to Follicular Lymphoma
Tiffany: Something that was also talked about at ASH (American Society of Hematology annual meeting) in general is the idea of precision medicine and how it’s a relatively new approach to cancer treatment. Is precision medicine being used as an approach to follicular lymphoma? What are your thoughts on precision medicine?
Dr. Martin: It depends how much of a fan of science fiction you are. To some degree, we’ve always practiced precision medicine. What do I know about this lymphoma? What do I know about this person? What do I know about all of the different treatment options? How do I put it all together?
Over time, treatments are becoming more specific in some ways, so you can apply them under certain circumstances. Our ability to understand more about cancer changes with new technologies. We’ve always been trying to personalize medicine in the sense of sequencing the entire genome of a cancer cell and saying this is the right treatment for you according to lab testing, but we’re not there yet for follicular lymphoma.
Over time, treatments are becoming more specific in some ways, so you can apply them under certain circumstances.
Dr. Peter Martin
There’s one treatment, a pill called tazemetostat, which has modest activity but is generally well-tolerated. It’s an inhibitor of an enzyme called EZH2, which is mutated in about 20% of people with follicular lymphoma. It’s approved for the treatment of people with mutated EZH2 enzyme, but it’s also approved for people with wild-type unmutated EZH2 if they don’t have other treatment options. Realistically, it works reasonably well in both groups, so it’s a precision medicine approach, but you don’t necessarily have to have the mutation to use it. That’s the closest we have right now, but this is coming. It will continue to change and there are other examples where we’ll see more of that.
Long-Term Implications of Chemo-Free Treatments for Follicular Lymphoma
Tiffany: I know you can’t read the future, but what do you think the long-term implications may be for follicular lymphoma, specifically for chemo-free treatments? What I hear a lot is that I’m living with cancer, not that I have cancer. What do you see with that in terms of chemo-free treatments?
Dr. Martin: People with lymphoma want treatments that work and are well-tolerated. Whether you call them chemotherapy, immunotherapy, or something else, if it works and is well-tolerated, that’s already great. They also want options that conform to where they are in life.
Different treatments that work in different ways have the advantage of potentially allowing us to mitigate some of the short-term and long-term issues that can come up.
Dr. Peter Martin
Every year, we have more and more options available to us. We always try to pick the right treatment for that moment, thinking about the here and now. We also try to think about how what we do today impacts what the patient’s life is going to be like 10 to 20 years from now.
More than chemotherapy, these new treatments potentially have a lesser impact on the body in the longer-term setting. With multiple lines of chemotherapy back to back, people will get through them for decades without major issues but over time, it catches up. Different treatments that work differently have the advantage of potentially allowing us to mitigate some of the short-term and long-term issues that can come up. Having more options is always better.

Clinical Research and Follicular Lymphoma
Tiffany: Both of our backgrounds are heavy in research. I like to talk about clinical research anytime I do a program to get the point out there because oftentimes, people think that a clinical trial is one of their last resorts, they’re not at least getting a standard treatment, or they’re not getting treated at all for their cancer. I know that at Weill Cornell Medicine, you have a robust research program. What does your research program look like and how receptive are your patients to joining clinical trials?
Dr. Martin: I appreciate your disclosure that we both come from a research background, so people should take that into account knowing that we have those biases. The number one barrier to entrance into clinical trials is not patient refusal. It’s because they don’t know that there’s an opportunity. The real burden is having physicians let patients know that this is something that they could do, not patients saying that it’s something they don’t want to do. It’s us. We’re probably the bigger part of the problem.
There are different reasons why somebody might want to participate or not want to participate in clinical trials. They offer new opportunities to access new treatments that might be more effective or better tolerated. In some cases, that might be when other treatments have been exhausted, but in a lot of cases, it might be when a new opportunity has already been well-studied in another setting and you’re looking to apply it in a new setting. There are also some downsides to research, a little bit more of a hassle often.
Tiffany: I’m a proponent of decentralization. Patients can get labs locally without having to track things like that. I’m a little biased when it comes to clinical research, but I do think it has so many benefits, so I’m always promoting it.
Dr. Martin: It can’t be understated that historically, there have been a lot of questionable research practices that were not always in the interest of participants. The medical community on the whole has tried to grapple with this. We’ve got multiple committees, like hospital and patient advisory committees, which try to minimize that and make research as ethical as possible.
There are going to be some people who are distrustful, which is their prerogative. I’m never the person who’s going to twist somebody’s arm to participate in a study that they’re not comfortable with, but I’m also not going to shy away from proposing a study because I’m afraid that somebody is not going to go for it. That’s not respectful to their autonomy either. You propose every option that exists and talk about the pros and cons then people will decide what’s right for them.
Tiffany: Absolutely. I always say too that we don’t give patients enough credit. We always talk about patient education. They need to know that clinical trials are out there and they’ll be more than willing to make that informed decision themselves.
Fertility preservation is also a highly charged issue, but it’s like research: if you don’t talk about it, people don’t have the opportunity to consider it.
Dr. Peter Martin
Fertility Preservation and Follicular Lymphoma
Tiffany: Something that is less talked about in general when it comes to cancer is fertility preservation. An abstract I saw at ASH talked about fertility. For younger patients with follicular lymphoma, does that discussion come up? From what I saw from the abstract, a lot of patients don’t bring it up. They don’t want to discuss it. What has your experience been in terms of having a fertility discussion with your follicular lymphoma patients?
Dr. Martin: Fertility preservation is also a highly charged issue, but it’s like research: if you don’t talk about it, people don’t have the opportunity to consider it. It’s important from the physician’s perspective to ask people about where they are and what they’re thinking about, but it’s also something that patients should advocate for themselves if it’s something they’re thinking about.
In general, follicular lymphoma happens as we get older, but a significant number of people get follicular lymphomas while they are younger and some of those may be considering having children in the future. We’ll get away from the reasons why somebody might choose to have or not have children in the setting of cancer; that’s a whole other complicated discussion.
It needs to be discussed early so that we can think about all of the treatment implications now and longer term, and how we sequence things.
Dr. Peter Martin
Many big hospitals, including Weill Cornell Medicine and NewYork-Presbyterian, have fertility teams that help people consider all of their possible options and how to preserve fertility. Fortunately, even the chemotherapy regimens that we typically use in follicular lymphoma don’t have a major impact on fertility and a lot of the newer treatments have no impact on fertility. The caveat is that you don’t necessarily want to be treating the lymphoma when somebody’s pregnant, although that becomes necessary in many cases and, depending on the treatments used, it often turns out to not be a problem either. It needs to be discussed early so that we can think about all of the treatment implications now and longer term, and how we sequence things.
Tiffany: Thank you for saying that. That’s a conversation you want to have whether you are considering children or not. If you’re younger and considering children, advocate for yourself. If your physician doesn’t bring it up, don’t be afraid to bring it up.

Bridging the Gap Between Academic Research Centers and Community Hospitals
Tiffany: In my experience talking to patients, they don’t get their diagnosis until after they see their primary care physician or go to the emergency room for something different. Because you’re from a large research center, you have multidisciplinary teams, which many people don’t have. They could be going to their community clinic. Patients may not know to go to an academic research center because they haven’t been referred to a specialist. Do you work with any community clinicians or community hospitals? What are your thoughts on working with community doctors to bridge the gap?
Dr. Martin: Even in New York where we have multiple academic medical centers, the majority of people with cancer are managed outside of those academic medical centers, so that’s a reality that we have to acknowledge. I also work in Brooklyn a couple of days a month. People must have access to medical care in their community.
We have to bring better medical care to the communities that people live in rather than vice versa.
Dr. Peter Martin
It took me a few years to come to this conclusion, which is embarrassingly slow, but it’s probably not fair to expect people and their caregivers to travel a couple of hours to see somebody when they have access to medical care in their community. We have to bring better medical care to the communities that people live in rather than vice versa. There are a lot of excellent oncologists practicing in communities and that’s where the majority of people can and probably should receive their care.
One caveat I’ll say is that all of cancer is becoming increasingly complicated, so I don’t think that people should feel uncomfortable seeking a second opinion. I have friends who work in community medicine throughout the whole tristate area in New York and I work with them and help them to manage their patients. Telemedicine has made that easier as well.
This is where patient advocacy comes in. You have to advocate for yourself and speak up about it. If your physician is uncomfortable with that, that might be a sign that they’re thinking about more than your best interests. You’ll find that almost all academic oncologists will encourage second opinions. I certainly do that and help arrange them when I can.
It comes down to a balance. What do you get out of it, what do you have to give to get that benefit, and is it worth it?
Dr. Peter Martin
Key Takeaways from ASH
Tiffany: The ASH annual meeting is a big conference. Was there anything for you that stood out?
Dr. Martin: Bispecific antibodies are going to be a major part of treatment for follicular lymphoma. Exactly how they fit in, which line of therapy, and which combinations get used is interesting to think about. They’re not going anywhere for a while, so that’s pretty cool.
Another interesting study that came out was a late-breaking abstract looking at tafasitamab combined with lenalidomide and rituximab. This was a randomized controlled trial that showed that the addition of tafasitamab to lenalidomide and rituximab improved time to progression. Effectively, it doubled it compared to lenalidomide and rituximab, which is probably one of the more common second or third-line treatments for follicular lymphoma, so that’s a pretty significant benefit.
In some ways, it’s a no-brainer to say that’s something we should do. On the other hand, tafasitamab is a little bit of a hassle. People have to come into the clinic frequently for it, so it’ll be interesting to see where this plays out everywhere. I don’t necessarily know how I’m going to use that information. Again, it comes down to a balance. What do you get out of it, what do you have to give to get that benefit, and is it worth it? But it’s a strikingly positive trial.
Tiffany: I’m going to be following that trial as well.

Conclusion
Tiffany: Thank you so much for this engaging and insightful conversation. I always learn something on the other end of these educational programs.
Dr. Martin, thank you for taking the time to speak with us at The Patient Story. It was so good to catch up with an old colleague and know that we both have remained dedicated to improving cancer care and finding a cure. I’m optimistic about the future as long as we have physicians and researchers around like Dr. Martin.
Laurie, thank you for sharing your story. Lived experiences are very personal and I’m forever grateful to patients who are open and transparent because I believe that it helps the next person and the next patient.
It’s so important to be empowered so that you and your caregivers can make informed decisions about your care. That includes being educated about the latest treatment options for your cancer.

Thank you to The Leukemia & Lymphoma Society for their partnership. The Leukemia & Lymphoma Society is here for you with information about clinical trials, resources, and support.
Follicular Lymphoma Patient Stories
Hayley H., Follicular Lymphoma, Stage 3B
Symptoms: Intermittent feeling of pressure above clavicle, appearance of lumps on the neck, mild wheeze when breathing and seated in a certain position
Treatments: Surgery, chemotherapy
Laurie A., Follicular Non-Hodgkin Lymphoma, Stage 4
Symptoms: Frequent sinus infections, dry right eye, fatigue, lump in abdomen
Treatments: Chemotherapy, targeted therapy, radioimmunotherapy
Courtney L., Follicular Lymphoma, Stage 3B
Symptoms: Intermittent back pain, sinus issues, hearing loss, swollen lymph node in neck, difficulty breathing
Treatment: Chemotherapy
John S., Follicular Lymphoma, Stage 4
Symptom: Swollen lymph nodes
Treatments: Clinical trial, chemotherapy




