Julie’s High-Risk Multiple Myeloma Story

Julie’s initial symptoms were somewhat atypical for high-risk multiple myeloma.
She started getting food aversions, queasiness, and fatigue. She then visited her gastroenterologist who, after ruling everything out on his end, did some blood work, which showed that she was anemic.
After being sent to a hematologist, she got a bone biopsy and was eventually diagnosed with high-risk multiple myeloma.
She voices how she processed her diagnosis, how her disease progressed after different treatments and how she decided to be part of two clinical trials.
This interview has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make informed treatment decisions.
The views and opinions expressed in this interview do not necessarily reflect those of The Patient Story.
- Name: Julie C.
- Initial Symptoms:
- Queasiness
- Food aversions
- Lack of appetite
- Fatigue
- Treatment:
- Stem cell transplant with KRD induction therapy (Kyprolis, Revlimid, dexamethasone)
- Chemotherapy: D+PD (Darzalex Faspro, Pomalyst, dexamethasone)
- Talquetamab (clinical trial)
- Cevostamab (clinical trial)
It’s so important to take charge and have control where you can. You can’t control this disease, but you can control how you respond. You could become knowledgeable and make decisions.
Introduction
I have two adult children: a 30-year-old daughter and a 32-year-old son and a wonderful husband. We’re going on 34 years together.
We are hoping to spend several months in Florida, which I haven’t been able to do before because of my treatment. It’s a real pleasure.
I love to golf, walk, work out, and travel. Although I didn’t do too much of that recently, I hope to get back to doing that.






Pre-diagnosis
Initial symptoms
Early July 2020, I was feeling queasy. I had a lot of food aversions and fatigue. I thought it was my stomach so I went to see my gastroenterologist. He did an endoscopy and had me get an MRI. He ruled everything out on his end, but he did do some blood work. He was concerned about those results and sent me to a hematologist. That’s when everything started moving along toward getting me diagnosed.
They weren’t the typical symptoms, as most myeloma patients have bone pain and other things so I guess that’s a little odd. Something was off in my body. I felt like I had morning sickness, but I knew that wasn’t possible!
I really am so grateful that he ordered the blood work; the hematologist took it from there. He had me come back for a couple of visits for more labs and then he said I needed to have a bone marrow biopsy. At that point, I knew something was really wrong. I felt it.
High-risk multiple myeloma diagnosis
We went in for the results. My husband came with me. The hematologist told me I had multiple myeloma. I didn’t know what that was. I figured it’s a blood cancer, but I’d never heard of it.
My initial feeling was, “Okay, tell me what I need to do to get through it and get cured. I’ll do whatever I have to do.” Little did I know what was ahead of me.
He generally described what it was. I had lambda free light chain and I had 80% plasma cells. He didn’t get into too many of the specifics, but he referred me to a top myeloma specialist who happened to be about 30 minutes away from me.
My myeloma doctor went into great detail about my disease. He was writing on a chalkboard. The most important thing is t(14;16), translocation (14;16), and beta 2 microglobulin of 6.2. I’m saying, “What?” He says, “You have a high-risk disease,” which he got into a little bit.
He explained that people who have certain cytogenetics with the disease may progress quicker from their remissions, have a shorter duration of response, and the disease could be a little more aggressive. He explained all of that as it related to how he was going to treat me.


Reaction to the diagnosis
Of course, I did what you’re not supposed to do: Google everything. What is high-risk multiple myeloma? What does it all mean?
All of the terminologies you read on Google are not good because they talk about progression-free survival. What is that? “Wow, am I not going to make it? Am I going to have only three years? What does this all mean?”
I was fearful. I was fearful of what was to come. I needed to understand it.
I did a lot of research after that appointment. I went online — The Multiple Myeloma Research Foundation, the International Myeloma Foundation, The Lymphoma & Leukemia Society — trying to get as much information and learn as much as I could, which was helpful to me.
I wanted to have some control in a situation where you don’t have control.

Breaking the news to the family
My kids were actually living with us at the time because of COVID. I explained it to them. I said, “This is treatable.” I didn’t tell them it’s not curable.
My family’s been incredibly supportive. My husband, especially during the induction period, was with me at every appointment. He was understanding. He was totally, totally supportive. I’m very grateful for that.


First-line treatment
Discussing the treatment plan
My myeloma specialist explained the induction therapy. He recommended KRd: Kyprolis, Revlimid, and dexamethasone. Then I would go for a stem cell transplant.
Because I was high-risk, he was planning on a tandem transplant, which would happen three to six months after my initial transplant.
I trusted him. My sense was when you’re first diagnosed, there’s a standard regimen to have induction therapy. My impression was there were a couple of drugs you could change up. He said that regimen would be best for high risk and I trusted that.

Getting a second (and third and fourth) opinion
Incidentally, I did get a lot of opinions early on before I decided on going to Hackensack University Medical Center in New Jersey. I had a consultation from one of the big hospitals in New York. They agreed that was a good treatment plan as well.
I know myself. I need to get all of the information. I need to feel comfortable. It is just my personality, really wanting that reinforcement to know I’m making the right decisions.
During that first year after I was diagnosed, I probably had four or five myeloma specialists, very well-renowned people, where I had second, third, and fourth opinions. Whenever I had to change a drug regimen and make decisions, I wanted to get input.
There are a lot of options for myeloma. Everybody’s myeloma is different and it can be treated in lots of different ways.
Induction therapy
I started my treatment in October 2020. My last treatment for induction finished in December.
I started preparing for the stem cell transplant at the end of December.


Side effects from the induction therapy
I actually only had two cycles of induction. One of the reasons was that I was getting fevers. Whenever I had the treatments, I would always have a fever the next day.
The first one was like 102°F. I would take Tylenol and it would go down. Subsequent ones weren’t as high, but the doctor was concerned about that.
They weren’t sure what caused it. They think it may have been the Kyprolis but they weren’t sure.
I had night sweats with the induction. I just changed my pajamas often!



Preparing for stem cell transplant
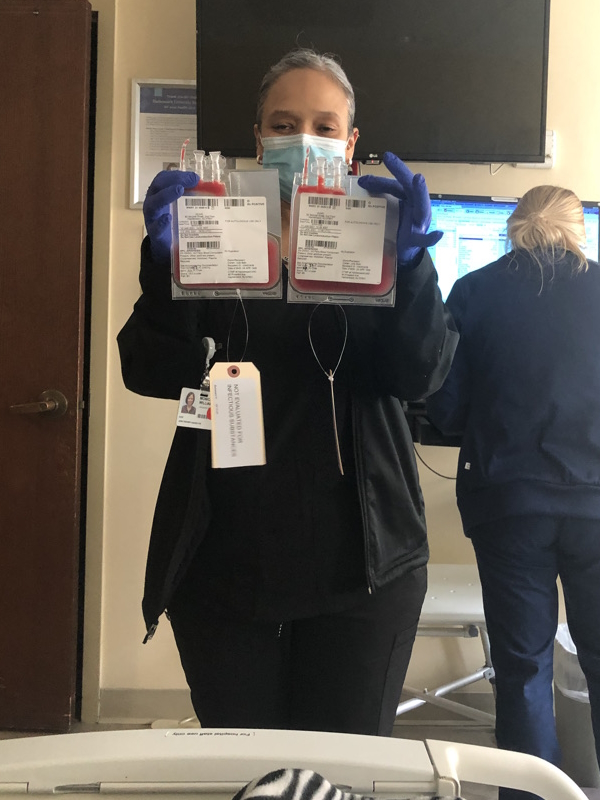
There’s a special stem cell transplant doctor who handles the transplants. It’s a whole process where they need to collect the cells.
In order to stimulate the growth of the stem cells, I was taking Zarxio probably eight or ten days in a row, giving myself injections. After, I had to get chemo.
They told me I would lose some hair in that process. I chose to get a short haircut at that time in order to ease that feeling of losing my hair.
They collected the stem cells. A week or two after, I was admitted to the hospital and they put in a central line.
I received melphalan, the high-dose chemo, the next day. I was admitted for about 12 days in the hospital.
Post-stem cell transplant
I ended up not having the tandem. I did not sustain remission after my stem cell transplant. My doctor said it happens to only about 10% of patients.
Three months after my stem cell transplant, I had progression of disease. I don’t know the medical reasons.
They weren’t going to proceed with a tandem stem cell transplant since I didn’t sustain the first one.

Reaction to the stem cell transplant not working
The first year after my diagnosis was really difficult. I didn’t sustain remissions and I understood that that was not like most people.
I was worried. Will I find something that’s going to work?
I was meditating. I was trying to become more spiritual. This was all during COVID so I was home. It was challenging emotionally.
Conversations with the family
Everybody was really supportive. My kids were fabulous. My siblings and mother live in different cities. They were always calling me to see how things were going.
I gradually started telling more people in my circle of friends about my disease. I would have Zoom calls with friends who I hadn’t talked to in months. There were a lot of positive things with that.
I established a relationship with a nurse navigator at the MMRF who was a godsend to me. She was always there for me to talk to if I needed to get another opinion or her thoughts about medications and whatnot.
I also have a myeloma coach who’s fabulous, who I initially spoke to quite a bit during COVID asking, “What could I do to pass the time?”



Second-line treatment
My myeloma team recommended a standard-of-care regimen, which was daratumumab, Pomalyst, and dexamethasone. At that point, I received a lot of opinions from other myeloma experts to really see if this should be the route. That was the route I took.
We started on that regimen. I was going to the hospital every week at that point.
They gave me Revlimid the first week because I had to get the insurance company’s approval for Pomalyst. For one week, I had Revlimid then they switched over to Pomalyst in that three-drug combination.
I had two or three cycles and then I started progressing again. Another decision point.


Mental & emotional state
I did respond initially but then the disease started progressing after that. Emotionally, I was scared. Will we find something that’s going to work?
I went for a consultation in New York City. I’m very fortunate to live in the New York City tri-state area with some top hospitals that are accessible to me. I know a lot of people in the country don’t have that. I was very lucky to be able to get in-person appointments with some of these specialists.
I went to Memorial Sloan-Kettering. The doctor there concurred with my doctor at Hackensack, agreeing on what the next thing should be. We talked about adding selinexor to the mix or going on a clinical trial. The decision point was shall I go on selinexor or go on a clinical trial.
They talked to me about the side effects of selinexor. I asked for their recommendation. The majority thought a clinical trial would be the best thing for me.



Third-line treatment
Exploring a bispecific clinical trial
It was the hot new area. A lot of developments and a lot of buzz. I didn’t know a lot about it. I didn’t understand immunotherapy or bispecifics but later it was explained to me.
I trusted my doctors. They thought the clinical trial was the best route. I did put trust in them.
I can’t say there was one thing that they said that made that decision for me other than feeling like they all felt it was the best thing for me to get on to a clinical trial.
At that point, Hackensack was trying to find me a clinical trial there, but they didn’t have anything available for me. One of the doctors on the team told me to talk to one of the top people at Mount Sinai, who’s still my doctor, about clinical trials.
I had a Zoom call with him and he said, “We have a clinical trial for you that you’re eligible for and it’s called talquetamab.” He discussed it with me, explained what the bispecifics were, and I ended up getting into that trial at Mount Sinai.


Joining the talquetamab clinical trial
You go through a screening process. You meet with the research nurses and the doctor. They let you know about the side effects. I was well aware of what those would be.
The screening was a couple of hours. They go through a lot of specifics with you about the trial and what’s involved. A lot of information. Then you sign a 35-page consent form.
My husband and I talked about it but not with the rest of my family.
I did have an idea about clinical trials from some myeloma support people. I figured five years from now, maybe I’d need one. I didn’t realize my disease would be that aggressive and moving so quickly.
I had spoken to other patients that my coach had put me in touch with who had the same cytogenetics as me. I wanted to reach out to people who had similar myeloma.
I did speak to a couple that had been on clinical trials and had myeloma for 20 years. Some of them were on the Revlimid trial and they talked to me about what that felt like.
I felt very comfortable participating in that way, that it could help other people and, hopefully, help me.



I started the testing at the end of August then I was admitted. You have to go into the hospital for the first couple of weeks for step-up doses so that was in September.
I was on it for two cycles and then my disease started progressing again.
For this particular trial, you stay in the hospital for 10 days. You’re admitted to the hospital because one of the side effects is cytokine release syndrome or CRS, which happens with these bispecific antibodies. Your body has to adjust to the drug and they have to treat you for the CRS in the hospital.
Experiencing cytokine release syndrome (CRS)
I had grade 1. It wasn’t horrible.
It happened the day after I received the first treatment. I had a fever and feeling like I have some sort of virus. They gave me tocilizumab. Immediately, the fever went away. It was a miracle drug.


Side effects of talquetamab
They told me that some of the side effects would be related to the skin, the hair, and the sense of taste. I experienced all of those side effects.
They had many patients on that trial in Mount Sinai. I had loss of taste and dry mouth. My fingernails split and fell off. The top layer of skin on my hands and feet came off.
It was difficult with those side effects. But I knew what the risks were. I knew I could possibly get them.
It seemed pretty quick. Within the month, the first thing I noticed was my hands got very opaque and then the skin started peeling.
The dry mouth came later. The loss of taste a little bit later. There was a little bit of a delay in the nails. They got worse gradually.
Nothing resolves them. They don’t go away.



Managing the side effects
The nurses were very good about giving me some over-the-counter things that worked.
There was a patient who was also on the talquetamab study, a woman about my age. She was willing to talk to other patients. We bonded.
She told me about creams, lotions, and things she used that helped. One was a prescription, Biotène for my dry mouth. There were some lozenges.
There was support there. The research nurses are all fabulous about giving you that information.


Bispecific antibodies for multiple myeloma
Talquetamab was twice a month. There would be a pre-treatment of Benadryl and Tylenol. I would get the medicine through IV, which would last a few hours. I stayed afterward for observation then went home. That was every couple of weeks.
It was a full day because when you start the day, first they take your labs and you have to wait an hour for the results. If your blood counts are good, they proceed. The pre-meds are an hour before you get the infusion so you’re waiting for that.
Basically, I looked at it as time to just hang out and watch the latest movies on Netflix. It was kind of a relaxing day.
Discovering plasmacytomas
When I was diagnosed in October, they found a couple of plasmacytomas from the MRI I had. I had a plasmacytoma in my pancreas as well as in my manubrium.
At the time, they weren’t sure it was a plasmacytoma on my pancreas. They weren’t going to do a biopsy; that was too invasive.
After my induction therapy, the tumors shrunk. They realized they were plasmacytomas most likely from the myeloma.
When I was inpatient for talquetamab, one of the side effects was very intense pain in my abdomen. The doctor explained that the medicine was targeting the pancreas because that was where I had this plasmacytoma, which I thought was fascinating.



Fourth-line treatment
As soon as I started seeing my numbers going up a little bit, I was alarmed. I wasn’t waiting till they got to the level they said is progression of disease. It was close.
I talked to my doctor. He recommended another trial, another bispecific called cevostamab. I asked for another opinion from another myeloma specialist and was actually considering going on a different bispecific antibody trial.
Each bispecific antibody has a different target. There’s BCMA, which probably many patients have heard about with CAR Ts. Talquetamab has a different target and cevostamab has yet a different target.
I was contemplating: should I go on a BCMA bispecific? Is that something I should consider?
My doctor at Mount Sinai said, “Look, there are only five slots for cevostamab at Mount Sinai. There’s one slot that opened up and you could have this slot.” He thought I should take it so I took it.
At that point, I was so uncertain about what would work so I went on the cevostamab trial.
Joining the cevostamab clinical trial
I don’t think I had to do all of the pre-tests because some of them were still good from the last study. They did require a brain MRI so I had that.
I was anxious to see if I was going to respond to this medicine. The only thing I was focused on was: am I going to respond? I was very anxious to see if it was going to work.
It was in December 2021. I was inpatient, but it was a different protocol from talquetamab. You’re inpatient three weeks in a row for only four days each time and then you’re discharged. They do step-up doses each of those three weeks, but you’re not there the entire time.


Side effects of cevostamab
I had CRS again. They treated that; it was fine.
I went home and I was on the schedule. This particular trial has an endpoint after 17 cycles. Each cycle is 21 days.
I was able to have three weeks in between treatments, which was wonderful. It was just great. The first time I had that kind of time span between treatments. We were able to go to Florida back and forth a little bit during that time and enjoy life a little bit so that was great.
COVID added some complications to that because I was fearful of traveling with my immune system the way it was. It was a wonderful feeling just being let out, being able to live my life.
Every three weeks, I went in for an infusion. There’s an endpoint to this trial. I think it’s the only trial where there’s actually an endpoint.
After 17 cycles, which actually equates to a year, you’re done with the treatment. That’s the protocol of this particular trial.
It was a pleasure not having to be at the hospital every week or every other week. Going once every three weeks, I knew that I had a full day there. I became friendly with the research nurses and all of the people there. It was my routine, but I was able to live my life in between that.
I also didn’t experience the kind of side effects I had with talquetamab. With cevostamab, I didn’t have any side effects where I felt badly. I felt fine.
My neutrophil count would come down and I had some neutropenia. I would have to have the Zarxio periodically throughout the treatment. I wasn’t tired. I didn’t realize it, but they were keeping on top of that.
During one of the cycles, they actually withheld treatment because of my white blood count. My neutrophils were low but that was it. Other than that, I felt fine through this process.
I was very grateful because I responded immediately to this drug.
Feeling hopeful
One thing I felt quite good about is the doctors said to me that these bispecifics act differently than other drugs. People are sustaining remissions. They’re long, durable responses. I felt that gave me some hope.
I actually connected with a woman who was on the cevostamab trial, one of the very early, early trials. She was off of the drug already, maybe a year and a half, and she was still in remission. I was hopeful hearing that.



Reassurance from another myeloma patient
It was wonderful. I found this woman from an article she wrote on one of the myeloma support websites and they were able to give me her contact information. She wrote about being on the cevostamab study and we had a phone conversation.
It was just reassuring. It’d be nice if people could reach out to each other on these trials, but I know there’s a lot of confidentiality there.
Final treatment & follow-up protocol
My final treatment with cevostamab was on December 1st, 2022. I’m in stringent, complete response, MRD negative, which I’ve been in since early spring. So knock on wood, I’m really hopeful and grateful.
I’m able to be in Florida. I just have to submit my labs once a month to Mount Sinai, go back for an in-person visit once every three months, and get a PET-CT. I’m very grateful right now.
Managing anxiety
I’m happy. I want them to be on top of it. I did ask my doctor recently, “Will I have to get the PET scans every three months indefinitely?” He wants to keep them going.
I have extramedullary disease and they want to make sure nothing is happening on that end that would show progression of disease. I hope to get to a point where I don’t need to go every three months for that, but I understand.
I accept the fact that it’s a chronic illness. I realize on an intellectual level that there could be remission. There could be periods where I fall out of remission. I have relapses.
Right now, emotionally, it’s hard to go there and I don’t want to think about that. I feel good now. I guess when that happens — if it happens — I’ll deal with it. I’ve accepted that intellectually. Hopefully, emotionally, I’ll be okay with that as well.




Importance of clinical trials
I am such a proponent of clinical trials right now. It’s a wonderful option for people that have an aggressive disease like me, who are not able to sustain remissions. I trusted my doctors that these were the best decisions for me.
Some trials are newer than others so they have less data. Cevostamab did not have as much data. I would ask questions about certain side effects and they just don’t know everything yet. I was told, cevostamab will be hopefully FDA-approved in 2025 so there’s still a way to go. One bispecific is already FDA approved.
There are so many options for patients. Early on in my diagnosis, I just didn’t really know if anything was going to work. I was worried that nothing would work for me and I am so grateful that I was on the trials. Unfortunately, one didn’t work, but I’m still happy that I participated.
You also get a lot more attention when you’re on a clinical trial. At Mount Sinai, you get your own private room.
It’s always an important conversation to have with your doctor. The clinical trials could mean the difference in your disease and getting better.
Words of advice
Everybody should feel comfortable getting second, third, or however many opinions they feel they need. If the doctor that they are seeing does not support that, I think that’s a problem.
I did tell my doctor I was getting these opinions as I was doing it and he was fine with that. I think everybody has to feel comfortable, whatever they’re comfortable with.
I always took half a Xanax before a bone marrow biopsy. It was quick. The good news is it doesn’t take long so it’s uncomfortable for a couple of minutes and then it’s over.
I personally like the doctor telling me what they’re doing so I’m prepared. It’s important. Then breathe.


I made a point to walk the halls almost every day when I was inpatient. In Hackensack, they have a completely isolated ward for the stem cell transplants where nobody was allowed in and maybe that was because of COVID. I was able to get out of my room and walk the hallways.
In the hospital, the days just went by. I had some Zoom calls with my family. I can’t even remember everything I was doing. I didn’t feel like doing a whole lot. Watching Netflix shows and so on.
I actually took a course on Coursera on how to analyze research studies, figuring that could come in handy for me with my diagnosis. It got very technical, but I did that.
It’s so important to take charge and have control where you can. You can’t control this disease, but you can control how you respond. You could become knowledgeable and make decisions.
I don’t think there’s anything wrong with getting second, third, and fourth opinions if that’s what you feel comfortable with. Doctors are happy to do that. They really are. Every doctor I spoke with gave me different insights.
Seek out all the information through all of the wonderful resources and nonprofits that we have for myeloma. There are a lot of people out there who want to help so I’m grateful.
I want to help people with my experience and I’m hoping that people can gain some knowledge from my experience.





Inspired by Julie's story?
Share your story, too!
Multiple Myeloma Stories
Clay D., Relapsed/Refractory Multiple Myeloma
Symptoms: Persistent kidney issues, nausea
Treatments: Chemotherapy (CyBorD, KRd, VDPace), radiation, stem cell transplant (autologous & allogeneic), targeted therapy (daratumumab), immunotherapy (elotuzumab)
...
Melissa V., Multiple Myeloma, Stage 3
Symptom: Frequent infections
Treatments: IVF treatment & chemotherapy (RVD) for 7 rounds
...
Elise D., Refractory Multiple Myeloma
Symptoms: Lower back pain, fractured sacrum
Treatments: CyBorD, Clinical trial of Xpovio (selinexor)+ Kyprolis (carfilzomib) + dexamethasone
...
Marti P., Multiple Myeloma, Stage 3
Symptoms: Dizziness, confusion, fatigue, vomiting, hives
Treatments: Chemotherapy (bortezomib & velcade), daratumumab/Darzalex, lenalidomide, revlimid, & stem cell transplant
...
Ray H., Multiple Myeloma, Stage 3
Symptoms: Hemorrhoids, low red blood cell count
Treatments: Immunotherapy, chemotherapy, stem cell transplant
...




