Refractory Myeloma Treatment
A Conversation with Rafael Fonseca, MD

Dr. Rafael Fonseca has been a practicing hematologist for almost three decades, now interim executive director at Mayo Clinic. As a veteran specialist of the myeloma field, he shares his insights on the latest in emerging treatments and clinical trial studies.
In this episode, Dr. Fonseca describes his typical treatment regimen for multiple myeloma patients who’ve become triple-class refractory, or whose disease isn’t responding to the three standard classes of drugs prescribed to myeloma patients: proteasome inhibitors, immunomodulatory agents (IMiDs), and monoclonal antibodies.
He delves into selinexor, melflufen, and belantamab mafodotin, as well as commonly reported side effects and how to manage them.
Thank you to Karyopharm for its support of our educational program. The Patient Story has full editorial control of our content.
The interview has been edited only for clarity.
Newer treatments
For that first relapse, we’re using a lot of the drugs that we mentioned during frontline. For most patients during the first relapse, you’re going to see one of the antibodies, something like either daratumumab or sarclisa, which is the other monoclonal antibody, isatuximab.
That may be combined either with IMiDs or with carfilzomib. A large number of clinical trials are being published and presented for this. A lot of what I’m going to say relates more to that second relapse more than the first one. That’s where a lot of this molecules would come into play. We can start with selinexor.
Selinexor (XPOVIO) Mechanism of Action
Selinexor is a very interesting molecule. It’s fascinating. It turns out that the cell nucleus of any cell has a little pump that gets out proteins that are going to be interfering with the division process of the cell in the transcription of genes.
Now, these proteins, you can think of them like chaperones. They don’t want to let things happen inside the nucleus. The cells sometimes need to get them out because if a cell needs to divide, then they don’t need some of those proteins.
These proteins, we know them otherwise as tumor suppressor genes. The reason they’re important is because if those proteins fail, then the cell can become cancer as well.
It turns out these proteins also could interfere with the whole process of cell death. If you get them out of the nucleus, you could enhance some of the activities of other drugs, but sometimes using it of itself.
That’s what selinexor does. It blocks that pump. It makes a cell more vulnerable and in doing so, then the cell is more likely to die.
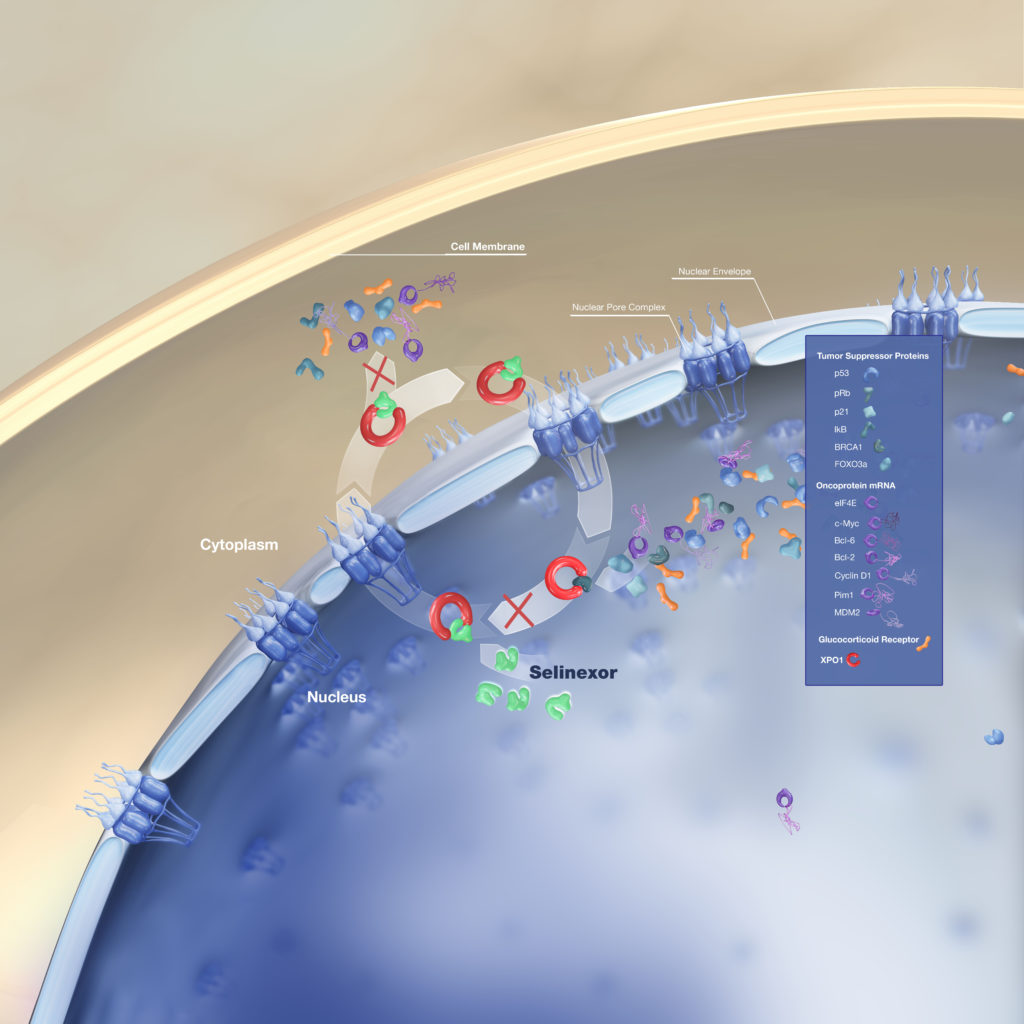
Selinexor in combination therapies
We’re seeing this both in combination with corticosteroids, with dexamethasone, but also in combination with other drugs. It has been tested and it turns out to be successful.
It was a large phase III trial that was first presented at ASCO last year, it’s called the BOSTON trial where selinexor added to bortezomib and dexamethasone showed benefit.
Now, I don’t use it a lot in combination with bortezomib. For patients who have advanced disease and for whom we’re looking for another option, this is one of them.
I combine it a lot with carfilzomib, which is the other proteasome inhibitor. We’ve learned a lot about this.
Determining dosage
Selinexor, I would say this was like the horse that stumbled at the start gate. It started first being used twice per week, and it was associated with pretty severe gastrointestinal toxicity and anorexia.
Since we have learned more about it, we use it now on a once per week basis with very good coverage with antiemetics, and actually patients are doing okay. This is one of the drugs, again, that is being used.
Now, for me, the bigger question will be is there a future somehow where something like this could be used perhaps at the lower dose as part of combinations even earlier on?
The one thing I think a lot about this, could this be something like dexamethasone? Dexamethasone enhances every other treatment that we use in multiple myeloma.
Maybe dexamethasone, plus some selinexor, future clinical trials might be the way to go. I don’t know. We need to see clinical trial data for that of course. It’s nice to know that it works and there’s a proof of principle for it.
New FDA approvals for Selinexor
That’s critically important. The BOSTON trial allowed one to three prior lines of therapy. That’s why it was approved as such with the package insert.
However, I think where I see it coming into place is going to be more into that second relapse and later. The reason for that is the first relapse seems to be pretty well-covered by some of the most active combinations.
There’s the CANDOR study, which was carfilzomib and daratumumab. The IKEMA study, which was isatuximab and carfilzomib, and then we have APOLLO and POLLUX, which are daratumumab with an IMiD, and with pom(alidomide) and with len(alidomide).
Pursuing best possible treatments ASAP
I think most people will stick for that first relapse but it’s really important to note the following:
You put your best foot forward from the beginning for the treatment of multiple myeloma.
We published last year a paper where we show a high degree of attrition with lines of therapy, such that you should not save the drugs for later. You do your frontline treatment and you do transplant.
If you do that, plus maintenance, okay, you’ve got one. Then at first relapse, you have to go with a good combination. That’s where potentially something like proteasome inhibitor and an antibody will come into play.
If you do that, what it means is that you could be seeing, again, progression-free survival of greater than two years after that first relapse. You need to have options that would be in the next line of therapy.
I think that’s a situation where something like selinexor can come into play and we’re going to talk about other options, as well. The reason I say this is because you should not hold back.
Give the best treatments upfront knowing that there’s going to be options down the line.
Selinexor side effects
Most commonly seen by Dr. Fonseca:
- primarily gastrointestinal (nausea, vomiting, lack of appetite)
- some myelosuppression
The main side effect has been gastrointestinal. There’s some myelosuppression. The myelosuppression is important that one needs to monitor, particularly platelets, but the reality is many clinical trials show that. I don’t think that is, in and of itself, a showstopper.
The major difficulty was with gastrointestinal toxicity. That came in the form of nausea, vomiting and lack of appetite. Now, the toxicity was much worse when we used to use selinexor in a twice per week schedule. Since the BOSTON trial, it’s been changed. Now it’s used at a once a week schedule and I can tell you what I do.
Most of the patients that I start on treatment, as I mentioned, I combine it with something like carfilzomib. It can be combined with pomalidomide or daratumumab, but I combine it with carfilzomib. I usually start at 80 milligrams once a week.
Dr. Fonseca Selinexor Regimen
Once a week = selinexor (80mg) + carfilzomib + triple antiemetic regimen
Managing side effects
I use a triple antiemetic regimen. What I mean by that is I usually start with something like ondansetron used before the first dose and then use it every six hours for a couple of days. Most patients we put on olanzapine, five milligrams at night time, plus the dexamethasone they get.
Dr. Fonseca’s Triple Antiemetic Regimen:
- Ondanestron before 1st dose, every 6 hours for 2 to 3 days
- Olanzapine (5mg) each night
- + Dexamethasone
You can improve that with the other antiemetics, the NK inhibitors, in particular Varubi is one that can be used, but you don’t have to do that from the get-go. This is the medicine that you just don’t prescribe, and then let go and say, “We’ll see you next month.” We have our nursing team call and monitor patients.
If need be, they can be supported with IV fluids or changes in doses or changes in the antiemetic, as I mentioned, but if you’re able to get through the beginning, then you can be successful.
I think this provides a new option for patients who would otherwise be very limited in what to do next.
Melflufen (PEPAXTO) mechanism of action
Melflufen is a new formulation and a more intelligent formulation, if you may, to the liver alkylating agents. We know how alkylators work well on myeloma. We know that from oral melphalan. We know because that’s what we use in the transplants and so forth.
The idea is that it can be used a bit more in a more specific fashion because of aminopeptidases in our body that would make it more prominent and more effective against the myeloma cells.
We are just seeing the start of it. We have the HORIZON clinical trials that show efficacy and that led to its approval. The real question is, what is the future for this, particularly in combination?
I can tell you I have a patient who has essentially exhausted all possibilities, and for a number of reasons, the patient has not been able to go into clinical trials.
We have looked at that recently as an option for him. We know that alkylators can work even in heavily-treated patients. There’s a brief, funny and famous story about a patient who lived here in the United States and had exhausted all possibilities.
She went back to Spain to see her family as one last event in her life. She was originally from Spain and she was sent to Dr. (Jesus) San Miguel. San Miguel looked at her record and he saw, oh, the patient has never gotten melphalan, so let’s try melphalan.
And low and behold, she responded very nicely to melphalan. The funny part of the story is that immediately she started calling the United States and says, “Hey, hey, there’s this new drug! It’s called melphalan. We should be using it for the treatment of myeloma.”
It brings color to the conversation. But it’s a good reminder that alkylators, such as we now see with melflufen, which has to be given intravenously, it’s used like an intravenous medication, just opens a new door for the treatment of myeloma.
Melflufen side effects
Most commonly seen by Dr. Fonseca:
- primarily myelosuppression
- some gastrointestinal (nausea, vomiting, lack of appetite)
With melflufen, we are paying a lot of attention, of course, to blood counts. Myelosuppression is the biggest thing as it’s possible with alkylators you can have some GI-toxicity but it’s not as prominent.
I think myelosuppression is the big thing. That of course is something we need to monitor because many of these patients have received extensive prior treatment. I don’t think we have a medical term for this but the bone marrow just becomes fatigued. It just gives out.
We have patients who have so much prior treatment that their ability to produce enough platelets and white cells, et cetera, becomes compromised.
Managing side effects
That’s just something to monitor. We have tools to support that and for the right person who has extensive involvement of the bone marrow, this may be something ultimately as the disease gets under better control to help with those counts as well.
Belantamab mafodotin (BLENREP) mechanism of action
This is a very interesting drug. As a single agent it has one of the highest levels of activity against multiple myeloma and it’s a BCMA antibody that is conjugated with a warhead, which is the mafodotin, a tubulin disruptor.
What it does is gets by to the myeloma cells, gets internalized, and then that releases the mafodotin. It belongs to a Trojan horse approach category of drugs that we use for the treatment of cancers. It’s, again, proven to be very effective. It works in multiple prior lines of therapy-treated patients. It can work in pretty advanced myeloma.
Now, it’s been explored in combination with other agents. That’s the natural thing to do, of course. If something works then we start combining it and then try to move it forward.
Belantamab mafodotin side effects
Most commonly seen by Dr. Fonseca:
- keratopathy (corneal toxicity, inflammation of cornea)
- chances in visual acuity, or blurred vision
- photophobia, poor tolerance to bright lights
- pain/discomfort in the eye
There is a very unique and peculiar toxicity with this particular drug and that is corneal toxicity. It’s not completely understood, but something that happens and has been described with other similar agents in oncology is that patients can develop inflammation of the foremost part of the eye, what we call the cornea.
So they develop this keratopathy and this could present itself in many ways. There could be changes in visual acuity. In the more extreme situation, there could be some poor tolerance to bright lights, what we call photophobia or just the discomfort and pain that comes with that.
Managing side effects
This is reversible, but obviously, it’s an important toxicity because then it will take some time before it all comes back to normal. When it was approved, it came with a requirement that patients be evaluated prior to every infusion by someone who’s either an optometrist or an ophthalmologist who can do a thorough assessment for the person and provide information to the treating oncologist about their ability to proceed forward or not with that.
It comes with that REMS (Risk Evaluation and Mitigation Strategy) plan. People need to get certified to know how to use this, but again, this is something that would be more efficacious than potentially that naked antibody, meaning without a warhead.
My hope is that as we continue to use it, we learn better ways to do it in a way that is not toxic to patients.
That’s really the history of every single myeloma drug. We started with lenalidomide, 800 milligrams. Lenalidomide, we changed that. Dexamethasone, we cut back. Bortezomib, we went from IV twice a week to subcutaneous once a week, carfilzomib once a week.
There’s got to be a magic way that we can find so we can use more of this important drug.
How do you track these side effects
It is our requirement for our prescribing of it that they are engaged for each one of those infusions. I think it’s fair to say, too, because of what the toxicity is that there’s naturally some reluctance sometimes by patients of saying, “Boy, my vision.”
For some individuals, for the right individuals, it’s going to be a good treatment. People are spacing it out.
I think that’s probably the number one thing that is being done. If it works and you don’t happen to get it, which by the way, the majority of people don’t get it. I mean, there’s a sizable minority that gets it, but there’s a smaller minority that gets a more serious version of this.
You don’t want to be in that group, but the reality is most people actually do pretty well. That’s the context of what you need to consider in regards to getting the treatment or not.
How do you determine which therapy to pursue for each patient
Oh, that’s a $64,000 question, because a lot of this depends on prior treatment and patient preference.
Our life becomes somewhat complicated as we walk patients through the decision-making process. It takes a nuanced conversation and discussion with patients as far as what their preferences are and what to do next.
I would say that the main conversation so far has been between the belantamab mafodotin and selinexor, but of course now with melflufen, that comes into play.
I think a lot of this will prove itself as more clinical trials are reported with combinations of these agents, but right now our life is complicated as far as all of what we need to do and discuss.
