Relapsed Refractory Multiple Myeloma Treatments
Dr. Krina Patel, MD Anderson &
Dr. Muhamed Baljevic, UNMC
From new FDA-approved drugs and combinations to the latest in immunotherapy, there is a lot shifting in the landscape of relapsed/refractory multiple myeloma. The Patient Story is excited to present our latest event that features top multiple myeloma experts: Dr. Krina Patel, MD Anderson & Dr. Muhamed Baljevic, Univ. of Nebraska Medical Center (UNMC).
Transcript
- Treatment Decisions
- Discussing different kinds of multiple myeloma
- Deciding what treatment path to take
- Multi-drug combinations > fewer drug combinations
- Impact of COVID-19 on treatment decisions
- What helps dictate how to treat patients
- Giving best therapies upfront
- The constant state of flux in myeloma treatment
- Importance of clinical trials
- Latest Treatment Options
- Selinexor (XPOVIO)
- How selinexor works (mechanism of action)
- Approved for earlier lines of therapy
- How to deal with selinexor side effects
- Not associated with secondary cancers
- How melflufen (PEPAXTO) works (mechanism of action)
- More targeted melphalan against the myeloma cells
- Older, more frail patients may benefit
- Melflufen side effects & ways to manage them
- What is isatuximab (SARCLISA)
- How isatuximab works (mechanism of action)
- When to use isatuximab
- Isatuximab vs. daratumumab
- Belantamab mafodotin (BLENREP)
- How belantamab mafodotin (BLENREP) works (mechanism of action)
- When do you use BLENREP in particular?
- Direction of BCMA therapy
- Risk Evaluation & Mitigation Strategy (REMS)
- Ways to address BLENREP’s biggest side effects
- T-Cell Immunotherapies
- CAR T cell therapy
- When the myeloma becomes refractory to treatments
- Older patients have had great responses to CAR T
- Common CAR T side effects
- Mostly Grade 1 or 2 cytokine release syndrome (CRS)
- Grade 3 cytokine release syndrome (CRS)
- Intervening quickly for more serious CRS cases
- Bispecific T-cell engagers (bispecifics)
- Talking about a myeloma cure
- Off-the-shelf options
- The importance of seeing a myeloma specialist
- More from Myeloma Specialists
- Relapsed/Refractory Multiple Myeloma Patient Stories
Thank you to Karyopharm for its support of our educational program. The transcript has been edited lightly for clarity.
Treatment Decisions
Stephanie Chuang (The Patient Story): Hi, everyone, it’s Stephanie with The Patient Story, and I am so excited about today’s event, I’m so glad that everyone could join us. Today we are talking about treatment options for relapsed refractory multiple myeloma patients. And of course, this is for everyone, people who are diagnosed with myeloma and of course, the caregivers, the unsung heroes in all of this.
I want to stress that today’s event is different from maybe other webinars that people have been a part of. We’re going to try to take medical jargon out of this as much as possible. Of course, it’s a part of the conversation, but this is about humanizing what this means for everyone out there, because as we know, there are so many things to talk about in this space. First, I do want to say thanks to Karyopharm for their support of our educational program today.
Now, we will be asking our doctors today questions that have been generated also from the audience. But anything that doesn’t get answered today, we will try to do some follow up after. And I want to kick off today with an introduction of our special guests, our top myeloma specialist today, Dr Krina Patel from M.D. Anderson and Dr. Muhamed Baljevic from the University of Nebraska Medical Center. Welcome.
Dr. Muhamed Baljevic: Thank you for having me.
Discussing different kinds of multiple myeloma
The Patient Story: So as we go into this discussion, again, we have a lot of ground to cover. I’d like for you to set the stage a bit, you know, because in terms of multiple myeloma, it’s unfortunately not curable. It’s treatable, and especially with more and more FDA approved treatments that are coming out constantly.
I think it’s important to establish that we have different kinds of patients here. You’ve got the early relapse and the late relapse, the standard risk, high risk. I mean, there are a lot of different buckets. So for this general discussion, I think we’ll be covering more of say the, after a few lines of therapy type of patients. But how would you categorize the different buckets in terms of when you look at a patient, how do you decide which bucket to go to first in terms of treatment path? And we’ll start with you, Dr. Baljevic.
Deciding what treatment path to take
Dr. Muhamed Baljevic: That’s a really good question and important question for us in general. Multiple myeloma, as you point out, unfortunately, as of yet still is incurable malignancy.
I will add that hopefully every decade brings us closer to the cure. And it’s a lifetime worth spending in search of it, I would say.
But you’re absolutely right. There’s many different things that we consider when we are looking at patients with multiple myeloma, particularly as their disease relapses, which in the vast majority of patients, unfortunately, it does. I would say that we all have probably n of 1 or n of a few where patients may be in a very, very long, durable responses.
Some of them actually might even be off all therapy. But it’s important to know that that was an exception and not the rules. So when it comes to understanding of how we approach relapsed and or refractory myeloma, different things play a part, really. Duration of initial response defines biology.

Multi-drug combinations > fewer drug combinations
Dr. Muhamed Baljevic: In general, we like to think that multi-drug combinations are better than fewer drug combinations, for example, triplets rather than doublets or in the newer times, even four drug combinations, rather than fewer than that.
When it comes to prior drug exposure and refractory status or toxicity with prior drugs, that’s usually something that helps us decide specific drug in combination that we might want to move forward with:
- age
- frailty
- comorbidity of patient
- patient preference
- and their goals of care
- logistics of drug administration. So is it oral, is it IV, is it subcutaneous? That helps us decide dose and schedules of treatment.
And then when we talk about the highest risk standard, that’s also extremely important and also whether or not they are transplant eligible, have they received transplant, et cetera, helps us decide oral treatment, approach and goals.
When it comes to prior exposures, we like to pay attention to any presence of peripheral neuropathy, for example, in which case we might want to avoid drugs like bortezomib. Any cardiac or heart comorbidities or diseases or failure, in which case we like to avoid drugs like carfilzomib or anthracyclines.
Whether or not they have renal insufficiency or impaired renal function, in which case we often need to adjust dosing of some of myeloma medicines, such as lenalidomide, whether or not they have had reactions to these drugs like rashes, et cetera. And in case those were severe, then that may really make us hesitate in terms of choosing some of the newer examples within those classes.
Impact of COVID-19 on treatment decisions
Dr. Muhamed Baljevic:
What’s important to note, of course, is we live currently in unprecedented times of the pandemic.
As we mentioned before, the modes of administration, the frequency of visits and monitoring, et cetera. We have to recognize that all oral regimens are more convenient and can be delivered for patients while they take their medications at home. But compliance needs to be monitored.
As you mentioned, IV infusions, they do require clinic visits, but there’s a question about a frequency of administration, which is an important factor, and also some medications having subcutaneous version that’s available instead of the intravenous one.
Lastly, we have to monitor any potential toxicities, including hematologic toxicities or any other intolerances that can come in, particularly with some of the newer agents. And all of this put together really gives us a broader picture in terms of how we approach every single patient individually and what decisions we may make for early relapse and particularly late relapse.
The Patient Story: Thank you for summing all that up, I know it was a lot of ground to cover. And by the way, we will be delving into side effects and management later on in the conversation, because I know that’s obviously so important for people.
Dr. Patel, do you have anything you want to add to that? And also, if you want to segue, too, into how and when you decide to restart treatment for a patient. What are the factors there? You know, examples are going from MRD negative to positive or symptoms or M proteins rising to a certain level. You have the floor.
What helps dictate how to treat patients
Dr. Krina Patel: Thank you. I think Dr. Baljevic did a great job of telling you all the different things that we look at for each patient to make sure we have the best outcome for that patient that’s in front of us.
To make it a little simpler and summarize, I think when my patients come to me, I saw 25 patients yesterday and each patient I don’t get very much time with all of them, but with each patient we see how they’re doing. And really our goals are for most patients I think, and most people in general, are you want to live longer and we want to feel well while we’re living longer.
We want good quality of life.
That’s really why we look at all of that. So for my patients, when they come in, we look if they’re high risk or standard risk, because that can change what I look at in terms of the M protein or MRD, et cetera. It can be defined differently and it can mean different things for high risk versus standard risk patients.
Then I look at patients to see if they’re frail or fit and if they’re frail because of the myeloma. That’s different than if they’re frail because of the comorbidities Dr. Baljevic just talked about.

Giving best therapies upfront
Dr. Krina Patel: That’s how we decide on all the different treatments that are available. What’s the best next step? I think the guiding principle is that the earlier you are in treatment, no matter what line of treatment it is, but the earlier you are, we have more options because your myeloma is not as resistant.
Our goal is to give you those best therapies up front and then we kind of use other things down the road. But as new research comes in, we actually get better therapies down the road.
And that’s the nice thing about myeloma, that we have so many new things coming in all the time that really you can actually get better responses later than something that maybe didn’t work as well earlier because we have so many options.
The constant state of flux in myeloma treatment
Dr. Krina Patel: We have these guiding principles, but things are constantly changing. That’s the other thing to think about. And so in terms of when do we change therapy right. So, of course, we have the IMWG criteria that everybody uses.
But an application, if I have a patient that’s really slow growing myeloma, that is doing really well on their therapy and their M protein is going up by 0.1 every six months, I’m probably going to watch that patient until their M protein gets to one maybe instead of the zero point five that we usually say is a biochemical relapse. So if someone gets to zero, they get to 0.5.
That’s biochemical progression as long as they don’t have any of the CRAB criteria. So the calcium, the kidneys, the anemia, the bones, right? So if they’re really slow growing and I know they’ve done really well for a long time on other therapies, I’ll just watch it.
And then once I get to one, maybe I’ll say, OK, let’s go ahead and switch therapy now versus my high risk patients. When I start seeing that M protein start going up, I’m watching them very closely. And the second they get to point five, which could be in a month, maybe two months, we switch therapy.
Really, it’s about the standard of care therapies that are available versus research trials that are available. And in the end, standard risk, high risk, we want to get you to the best therapy we think is there for you. But my higher risk patients, I tend to do more research trials earlier because we know that therapies we have already still don’t do as well as they do for standard risk patients.
Dr. Muhamed Baljevic: In my clinic, I have two goals. One goal is to try to prevent myeloma from shortening people’s lives. And if left unchecked and untreated, that’s invariably what’s going to happen for some patients, sadly, very rapidly, for some later.
But invariably, people don’t live with hematologic malignancies, myeloma or other without major effect on their livelihoods. And to while we do anything that we decide together with patients what we will do, that we do it in a way that really allows them good quality of life and that it meets their goals of care, and that we respect their idea of what it means to have a cancer and what they wish to pursue in terms of our best efforts to suppress it, eradicate it if we can, as we said, very challenging with myeloma.
But at least having patients be in a position where their disease is not dangerously influencing their life or, as we said, shortening their life while at the same time allowing them to to live it with a good quality and with dignity. There’s many other things, quite frankly, to consider. I think that the principle Dr. Patel explained in terms of high risk standard is a really quite important, very, very important.
Importance of clinical trials
The role of clinical trials and participation in clinical trials is of major value, not just in myeloma, but in any cancer.
Quite frankly, as I started sort of my working as an independent physician some years ago, the analysis that I took a look at when it comes to myeloma and other cancers by the mechanism of action, the new regulatory approvals that we have seen in myeloma over the last number of years is really only lung cancers rivaling that.
So we are very, very busy. And that’s good, conducting new clinical trials and trying to find solutions that bring better answers for all these phases of disease and all these situations. And that’s really a discussion and a conversation that we always have to have with our patients.
Latest Treatment Options
Selinexor (XPOVIO)
The Patient Story: I love how both of you have talked about access to this care, the newer treatments and also quality of life, because we hear it talked about. But when you talk to patients, obviously, you really understand the impacts to day to day living. And that’s a huge consideration. It’s yes, I want to extend my life, but I also want to be able to live it to the best of my ability.
Dr. Baljevic, you talked about these different mechanisms of action and we started to see some newer ones being introduced and approved. So let’s go ahead and start talking about some of these. I’d like to start with selinexor, which I know at first it was for patients who had had at least four previous treatments or lines of therapy. And then now with the bortezomib and dexamethasone it’s at least one prior line of therapy, which of course means very different situations.
And you can have different experiences. But let’s open it up to you, explaining the mechanism of action, like how it works in layman’s terms and anything else about what you’ve seen with selinexor?
How selinexor works (mechanism of action)
Dr. Muhamed Baljevic: Selinexor is just one in the line of the new agents that have entered the multiple myeloma treatment space. Selinexor, or XPOVIO, is a first in class oral selective inhibitor of nuclear export.
So in layman’s terms, what that really means is if we just take a step back and look at how our bodies are made, they’re made of cells right? And each cell, has a membrane, has a sort of internal compartment. The liquid compartment has a nucleus where all the genes are, et cetera, where major processes are happening. So they are pores in the nuclear pore that allow transitioning of various proteins and molecules inside of the nucleus and outside.
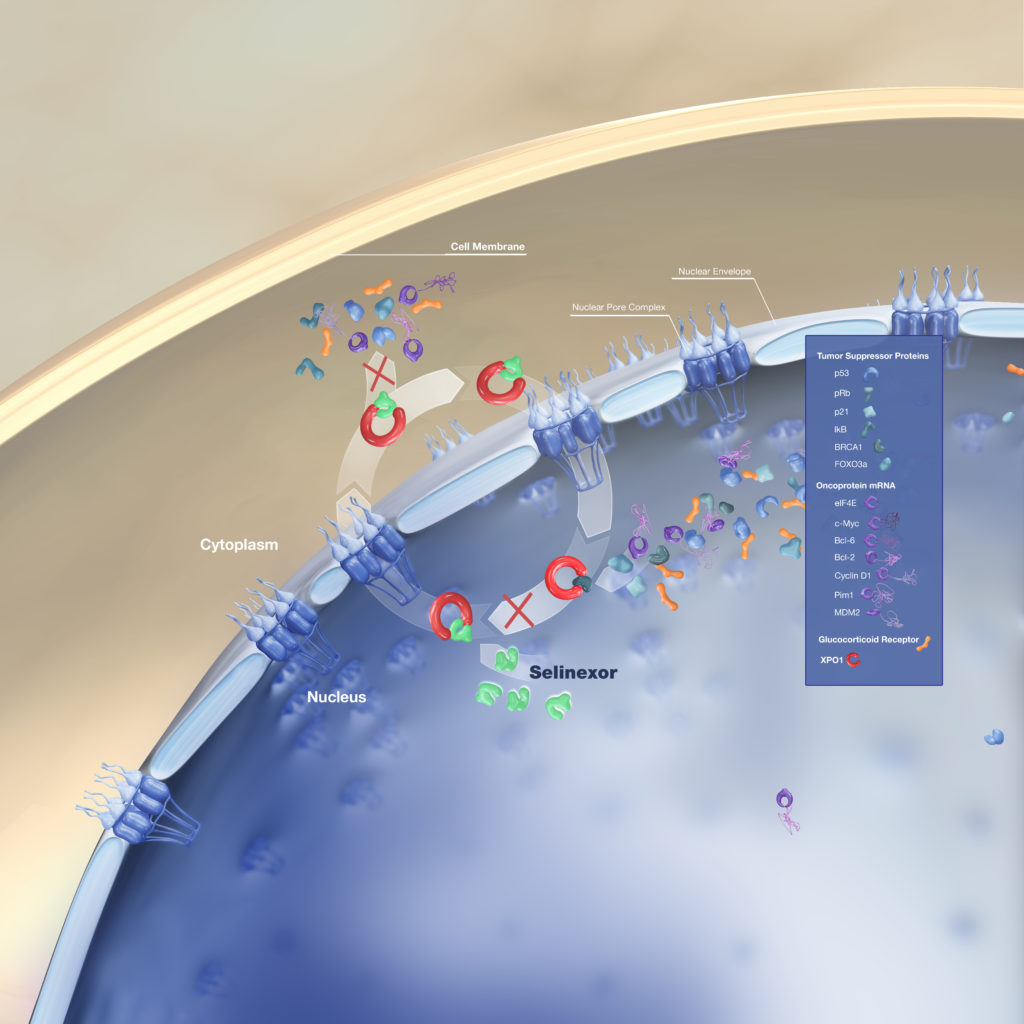
What selinexor does is it is a selective inhibitor of one of those nuclear pores exporting one or XPO1, which is a major nuclear export protein for a variety of really essential proteins in the biology and the life cycle of any cell. So that includes tumor suppressor proteins.
That includes proteins that are called oncoproteins, which are in general terms proteins that stimulate division and may cause the cancer to happen. We know that this XPO1 is expressed in myeloma.
And the high levels of XPO1 levels enable escape from these tumor suppressor protein mediated cell cycle arrests and apoptosis, which is a process by which a cell dies.
We also know that XPO1 levels correlate with poor prognosis and drug resistance in multiple myeloma. So then when we look at what selinexor really does for patients and against their myeloma disease is it reactivates multiple tumor suppressor proteins by preventing nuclear export.
It inhibits oncoprotein, meaning the bad protein that we mentioned earlier, translation meaning getting it to the active phase. And then it also works synergistically, meaning it helps, right. It works with the glucocorticoid or steroid receptor signaling in the presence of dexamethasone.
Approved for earlier lines of therapy
Dr. Muhamed Baljevic: What you alluded to earlier, selinexor was approved several years ago, initially following the STORM trial, which described the selinexor use in twice weekly setting, which, truth be told, was very, very challenging. It resulted in multiple side effects, namely GI side effects from the standpoint of symptoms and also some side effects in terms of lower cancer myelosuppression that normally physicians follow for their patients.
However, since then, we just had recently published data from the BOSTON Phase Three trial that looked at combining selinexor with bortezomib and steroids and compared that to the treatment with bortezomib and steroids alone. That Phase Three trial showed a very meaningful progression free survival benefit with this triplet combination versus the combination.
But one of the most important things that that trial taught us and showed us is the fact that we can use selinexor on a once weekly schedule rather than twice weekly schedule.
And in fact, some of the follow up studies, including the STOMP study, which is a multi-arm, more than 10 arms of different selinexor combinations with different modern classes of drugs like carfilzomib, which is a newer cousin of bortezomib or pomalidomide, or even some of the newer drugs that we’ll get to probably very soon.
So all of these arms in this trial are also demonstrating that, in fact, use of selinexor on a weekly schedule is a, first of all, safe and feasible. It appears to be and it’s also fairly effective. So I look at selinexor maybe as a drug that’s been described by some as a horse that stumbled out of the gate and now we are learning.
And in fact, that’s the whole history of myeloma. Quite frankly, if you look at what we’ve done in the history of this malignancy, we have learned not to… We initially used very high dose steroids and now we learn not to do that. We have used bortezomib as an intravenous drug, now we use it as subcutaneous.
Earlier immunomodulatory drugs, we used very high doses of thalidomide. But now we have lenalidomide and pomalidomide. And even in daratumumab, our antibodies, we used to use it intravenously. Now we have a subcutaneous form. So we always learn, right?
And we are learning rapidly with selinexor. I think it’s a very important drug, in my opinion, because it offers, first of all, novel mechanism of action. That’s very important for our patients, because one of the cornerstone principles of treatment of myeloma is the fact that this is a disease that generally benefits from a combination of therapy rather than single agent therapy.
That’s not to say that always adding more is better. There are certain thresholds at which you start observing more toxicities rather than gaining benefit.
But the principle of long term management of myeloma really asks of us to having newer drugs with newer mechanisms, the ones that myeloma cells have not seen, the ones that they may presumably not be resistant to, that they may be more sensitive to.
Selinexor definitely offers that. And as we continue pursuing some of the additional studies, as I mentioned, we are going to continue learning even better ways of utilizing a drug like selinexor. And in my clinic, I do depend on it for patients with multiply relapsed refractory myeloma.
How to deal with selinexor side effects
Dr. Muhamed Baljevic: I also think it’s very important and we will hopefully provide some educational materials for the viewers as well to look at how we manage and prevent as much as we can side effects that can be a result of selinexor treatment.
That, as we mentioned, is GI toxicities, nausea, vomiting, diarrhea, and also cytopenias, reductions in the counts. So whenever I use selinexor either as a standard regimen, I like to use it, obviously, as we said, on a weekly basis and usually in combination with some of these newer generation proteasome inhibitors or immunomodulatory drugs like carfilzomib or pomalidomide.
The arm with the daratumumab combination has already been published and showed efficacy. I like to use it with a proactive stance towards management of the side effects. So this is not a drug that we give to patients to tell them to just go home and come back in a month. A week we check in and then we ask them to be proactive about letting us know how they feel.
I usually provide prescriptions to all of these, supportive care medications such as Zofran, of course, always needs to be part of antiemetic medication. We always ask patients to take dexamethasone before the selinexor because it helps. Olanzapine is very commonly something that I give and will ask patients to use in general.
Sometimes I may even use growth factor supports for even white cells or platelets if we need to, to try to support us or prevent severe reductions in counts, which would otherwise you know prevent us from treating patients or make us reduce the doses which would have compromise on the efficacy.
Not associated with secondary cancers
Dr. Muhamed Baljevic: So all of these are important things when we are thinking about it. The last thing I will mention about selinexor, what I think is also exciting, is that it’s not associated with, as far as we know so far, obviously it’s a new drug, but it’s been on the market and it’s been in investigation a number of years.
As far as we know, it’s not associated with risk of secondary malignancies, with the risk of rashes, for example, and may potentially lend themselves as an agent of choice as we start tackling progressively more and more some of these pre malignant conditions with any of myeloma agents.
So when you have an agent that doesn’t risk, like, for example, alkylating agents or even immunomodulatory drugs are known and associated with some of these risks, that may potentially be useful.
Dr. Krina Patel: I think that was perfect. I think the biggest learning points are the lower dose. The once weekly dose works a lot better, proactively giving two antiemetic or two nausea medicines ahead of time and potentially even a third one.
Most of my patients get fluids once a week, at least the first month to make sure that they don’t get dehydrated, especially in the summer in Texas right now. So we kind of do a lot of proactive things to make sure that they do well without those side effects. And then it’s just monitoring.
Usually after that first month, people start doing better with that, even if they were having side effects. So that’s what’s a little different, too, is that unlike other medications where we start to see side effects long term here, those GI side effects tend to actually improve, which is good.
But we want to make sure people’s weights are doing okay, et cetera. So it is a little bit more monitoring that first four weeks, but then it becomes a little bit more like clockwork.
The Patient Story: We like to hear clockwork, some sense of what to expect is always nice, I think. Wrapping this up, I know it’s given orally and that’s another big topic right. And Dr. Baljevic talked about how there have been iterations, too, on whether it’s IV to subcutaneous like a shot or oral pill. And I’ve talked to many patients, who of course are like, yes, please, I want the pill, give me that. Thank you very much.
How melflufen (PEPAXTO) works (mechanism of action)
We have three more of these to get through and that I’d love to be able to get to the CAR T and the bites. So let’s go ahead and go to melflufen, which has been FDA approved with dexamethasone for relapsed refractory patients with four more lines of therapy. Dr Patel., if you want to describe that, that’d be great.
Dr. Krina Patel: Melphalan is the drug we’ve had the longest for myeloma besides steroids. And here you’re actually repackaging it to be bound to peptide that helps it get once it gets into the myeloma cell, these aminopeptidases that are in the myeloma cell help break it down so that then the melphalan can actually do what it does, which is cut the DNA like a regular alkylator, like melphalan does, that when you cut it, it can’t survive.
So it’s using an old drug, but getting it to the myeloma cell. So you’re actually targeting it into that myeloma cell compared to regular melphalan when you get an IV, it sort of goes everywhere and it goes into all the cells that are dividing faster in your body. So toxicity tends to be higher.
More targeted melphalan against the myeloma cells
That’s really the thought process that here you get more melphalan concentrated into the myeloma cell activated in there, which kills more myeloma cells and hopefully gives you less toxicity.
In general, it has actually been very well tolerated for patients.
Older, more frail patients may benefit
Dr. Krina Patel: Once again, melflufen plus dex(amethasone), it’s considered a doublet, so likely not the best regimen. But I do think for patients, especially my older patients who are more frail, who couldn’t go through a stem cell transplant or couldn’t really get alkylators,
I think it’s a great substitute for alkylator therapy in myeloma. And I know there’s been other trials that have been going on. There have been some safety issues. So I think just like all other therapies in myeloma, we have to kind of let that play out first and really see which patients that it’s probably the best for. But I do have a patient population that I think really having a more targeted alkylator would be beneficial for.
Melflufen side effects & ways to manage them
The Patient Story: Ok, thank you. And anything I know you said generally tolerated pretty well, but anything you want to point out in terms of side effects and management that you tell your patients?
Dr. Krina Patel: Counts are one of the big things with alkylators in general, so platelets and white counts, so infections in case your white count is low et cetera, neutropenic fever.
And then their GI toxicity actually wasn’t so bad. You would expect with melphalan to have really bad diarrhea, nausea, vomiting, and it was actually pretty well-controlled. So nothing else that I can really think of that was out of the ordinary.
What is isatuximab (SARCLISA)
The Patient Story: Thank you so much, Dr Patel. We’re going to switch over to isatuximab now. And Dr. Baljevic you know, we know that it’s been approved for various situations.
So with pomalidomide and dex for patients with at least two previous therapies and then with carfilzomib and dex for patients who’ve received between one and three. So I just wanted to get that out of the way. What would you like to say about isatuximab and how it works and when you decide to prescribe it?
How isatuximab works (mechanism of action)
Dr. Muhamed Baljevic: Of course. So, as you mentioned, isatuximab is another example of a monoclonal antibody that targets the CD-38 receptor on the surface of the myeloma cells. It binds to this specific epitope of the CD-38 protein molecule resulting in anti myeloma effects through several mechanisms which include:
- antibody dependent
- cellular mediated cytotoxicity
- complement-dependent cytotoxicity
- antibody dependent cellular phagocytosis
- and direct induction of apoptosis mechanism similar to those reported for other monoclonal antibodies.
So this was really a mouthful, but basically various different ways of engaging immunity and causing death and toxicity to the myeloma cells, either directly as an antibody or by sort of calling your colleague, help us from the immune system to try to attack the myeloma cell.
When to use isatuximab
As you correctly mentioned, isatuximab was recently tested and the results of people with ICARIA and IKEMA studies were presented. And as you also correctly pointed out, in different patient populations, I would, rather than going through really specifics of sort of the overall response rate and toxicities, et cetera, what I think is important to know is the following.
A mounting amount of data, an increasing amount of data is suggesting that incorporating CD-38 antibody directed treatments to myeloma patients is leading to benefits, the overall response rates and the length of time in which, whatever the response may be in the end, which by and large is very, very deep you know responses, such as stringent complete responses and also significant proportion of MRD negative responses.
So we know that incorporating CD-38 targeted therapy with monoclonal antibodies is very good and that it brings a huge benefit to treatment of myeloma.
And this also brings up another question of sort of your best foot forward, right?
When we want to try to treat patients, we always try to give them the best treatment when they are sort of the youngest, when they are the fittest, when they are least tired, if you want to say, from various treatments when their bone marrow is least damaged over time, etc.. So that’s really important.
Isatuximab vs. daratumumab
Two, daratumumab was the first antibody that targets CD-38 that was approved and is an antibody that we have the most experience with. It’s been on the market for far longer than isatuximab, but isatuximab is also coming up.
So the two studies that you mentioned are examples of studies in relapsed refractory myeloma, either in combination with pomalidomide, with IKEMA or with the carfilzomib, with ICARIA.
These are examples of newer generation drugs that bring significant benefit to our patients relapsed refractory disease. My last comment on sort of what this means is really to do with always knowing what the data is exactly telling you and showing you and in which patient population was it studied? So, for example, the isatuximab and pomalidomide developed by exposure to POMALYST.
So if you have somebody who’s progressed on POMALYST in refractory, maybe that might not be the best combination to think about. Similarly, isatuximab carfilzomib, a trial looked at patients who didn’t previously get carfilzomib. So these high response rates are really contingent upon understanding who that benefit is for.
And does that really apply to your patient? These are fabulous results. Isatuximab has been approved as a result of both of these studies and is probably going to increasingly be incorporated into the treatment paradigms.
There are some major differences, for example, between these two antibodies. Daratumumab does have availability of subcutaneous dose. But perhaps the pricing of isatuximab appears to be a little bit more favorable to daratumumab.
We need bigger studies to really prove this. But there’s there’s points about both of these and pricing, the way you administer it, et cetera, that really need to be taken into consideration. And which one of these combinations would you isatuximab or daratumumab combinations with any of these other agents.
It really has to be individualized decision for every patient of yours. And sometimes you will make a decision that may rely more on what the patient preferences are.
And that’s okay as long as you sort of educate your patient on what would be perhaps the most beneficial regimen in that exact moment for their specific disease.
The Patient Story: love that message, it’s obviously tailoring to each person because this disease appears in so many different ways for so many different people. Dr. Patel, the last one in this category you want to talk about is the belantamab mafodotin a.k.a. BLENREP.
Belantamab mafodotin (BLENREP)
So the FDA has approved it for patients with four more prior lines of therapy. And I’m generalizing this to be broad strokes again. But Dr. Patel, if you could describe that mechanism of action, a.k.a. how it works and when you decide to prescribe it.
How belantamab mafodotin (BLENREP) works (mechanism of action)
Dr. Krina Patel: This is our first BCMA directed therapy that we have for myeloma. Once we found BCMA or B-cell maturation antigen, this is a flag that’s on myeloma cells and not on any other major organs or tissues.
It was a huge discovery. Basically, everybody started attaching anything they could to that BCMA to get it to that myeloma cell to kill it.
So what we talk about next with CAR T and bispecifics, same thing, we’re using that target BCMA to get some type of drug or immunotherapy, et cetera, to the myeloma to help kill.
And so here you have something called an ADC or an antibody drug conjugate. So you have the antibody for BCMA and attached to it, you have this drug that I won’t go into too much detail about how that drug works. But basically when you get the infusion, it’s an IV, when you get the infusion, it’s once every three weeks.
It goes in and it actually attaches to the myeloma cell, kind of like the anti-CD 38 antibodies do, but instead of CD-38 it’s BCMA and now this drug gets put into the actual myeloma cell. So kind of similar to that melflufen story, it just works a little bit differently of how it gets into the cell.
But once it gets in there, it actually kills the myeloma cell. And so the early studies once again were doublets, so BLENREP by itself, and that’s what’s approved in four lines of therapy. The response rates are very similar to single agent daratumumab, so in the 30 percent range and my patients have actually done really well on it when we’ve used it.
When do you use BLENREP in particular?
I tend to use it for my patients who might have a little bit more frailty or are older. And I really want to give them just a single agent; they’re going to do better with that. And I know that they have a good chance of response. Those are my patients that I really put on there initially.
The nice thing about it is that they have done studies in patients with kidney problems. So kidney damage, which a lot of our therapies, unfortunately, we can’t use safely in patients with kidney problems or we have to be really careful when we use it.
And their studies actually show that even patients with 50 percent reduced renal function and their kidneys weren’t working as well, they still got great benefit from it.
I’ve actually used it in a couple of my dialysis patients recently and had some fantastic responses without major side effects. So those are things that we’re all sort of learning now as we try to use these drugs. But I think it really that group of patients is one of the unmet needs for myeloma. And that’s where I really like seeing what I see with BLENREP right now.
Direction of BCMA therapy
Having said that, the big question really is BCMA therapy. Can you use different ones, can use them in different order? If you use one, will it make another one in the future not work? So because of those types of questions right now, we don’t have answers for that.
So we’re a little bit more careful. And instead of giving everybody BLENREP, you know, the other part of it is it’s also high maintenance drug compared to similarly to what we’ve talked about already today in the sense that it can cause eye issues, which can cause these little microcysts in the eye that we call keratopathy.
And for most patients, they don’t have symptoms. But if it gets really bad, it can cause ulcers and all kinds of things that you don’t want.
Risk Evaluation & Mitigation Strategy (REMS)
So you have to see an ophthalmologist or an optometrist for an eye exam every single time you’re about to get the drug.
So that’s why I call it high maintenance, because you have to see me and you have to see the eye doctor.
We’re learning more about it, once again, and patients are doing better. They’re looking at giving it every four to six weeks instead. And patients still do just as well and maybe have less side effects. So those are all the things coming down the line.
They’re putting it with everything, all the different therapies out there for myeloma, which will likely increase that response rate and doing it sooner. So I think there’s a lot of exciting things coming down the road with it as well in combination. But even a single agent, it’s our first BCMA therapy and it seems to be pretty good for at least a subset of our patients.
The Patient Story: That’s great to know that, especially for the unmet needs category of people, that there are options that are coming out right. So it may not fit the bill for everyone. And I also appreciate you bringing up the topic of the issues with the eyes, because that is the most talked about side effect when it comes to BLENREP.
And that’s why if you as a patient or caregiver hear about the Risk Evaluation and Mitigation Strategy or REMS, Dr. Patel really spelled out for you what that really means, which is your oncologist is going to be working with the ophthalmologist or optometrist to make sure there’s constant communication about what’s going on with your body. So thank you so much for that.
As we wrap this part, is there anything else you want to talk about with side effects? I know that both of you have addressed the nausea and vomiting of the antiemetic sort of regimens that you like to give patients and not to chase, to give them beforehand.
By the time you’re feeling the nausea, usually is pretty hard to catch up with. And you’ve also talked about anemia. I think anything about neutropenia or loss of or decreased appetite or any other issues that you want to address in terms of how you advise your patients to minimize those toxicities.
Ways to address BLENREP’s biggest side effects
Dr. Muhamed Baljevic: Well, I want to really echo everything that Dr Patel said about BLENREP and just reinforce what you mentioned as well, which is the fact that we are hopefully going to learn how to maybe optimize the use of BLENREP. We talked about this before, so sure.
But many of us believe that perhaps dosing of belantamab can be stretched out a little bit without compromising the efficacy too much. And with these issues with corneal toxicities and such, it can be a really big problem.
keratopathy (eye/cyst problems)
And unfortunately, another thing to raise is we don’t have good treatments for this. You know, the SteriDrops were initially part of supportive measures, but really they don’t prevent or dramatically help. We do advise our patients to use artificial sort of moisturizing solutions for their eyes, et cetera.
But it’s really the sort of very, very proactive management that we have to employ with BLENREP. And as Dr Patel mentioned, I mean, what makes it easier is that it can be either ophthalmologist or optometrist as long as somebody looks and this is all done through the so-called REMS program that both or providers need to be registered through in order to administer this therapy. So I think another example, as we said, of a drug that we may potentially learn in the near future that could be used in slightly different ways, that can optimize the entire experience for patients.
Neutropenia
In terms of your question for neutropenia specifically, we talked about the monoclonal antibodies in some of these combination trials. Neutropenias have been sort of the various other cytopenias with neutropenias in general as well, have been showcased as a toxicity. And that just requires really active management and following and any myeloma treatment in fact, can cause neutropenia or anemia or reduction in platelet counts.
It’s a combination of measures of proactive management that those reductions, sometimes the growth factor supports are required in order for you to achieve the sort of the most optimal and the result.
I will also mention lastly, the approach is not the same in every single patient. We all have patients whose bone marrow is completely filled with myeloma, unfortunately, and then their cancer as a fact, as a function of impaired bone marrow function, which normally produces counts.
So sometimes you just have to treat through that with support of transfusions and of medications to try to clear out the bone marrow and try to allow it to work better.
That’s scary sometimes to try to treat through those types of difficulties, but it is sometimes necessary. So our approaches really do vary and can change from situations where we like to hold off, wait until the recovery, maybe support some, to all the way to you’ve got to treat through it in order to help the patient.
It’s a combination of different strategies.
The Patient Story: Absolutely not one size fits all. And this is you’re both highlighting why specialists are so important, because you have so much coming out and you need to have someone who, for the most part, especially when you get to more complicated situations, who really knows all the different options.
T-Cell Immunotherapies
Now, I’d love to switch over to the T cell therapies, which are really exciting with both CAR T and the bispecific T cell engagers or (bispecifics). So Dr. Patel, I’ll let you take it away.
Dr. Krina Patel: Awesome. Okay, so I promise this is not going to be lecture style. It’s really more for teaching and just to understand how these I mean, these sort of newer immunotherapies work, and specifically I’ll talk about CAR T cells and the bispecifics, which are some of the biggest things that are happening at all of our big hematology conferences, oncology conferences and on all the patient support, social media, etc..

CAR T cell therapy
I get things all the time from my patients that they found on different patient support channels that they have about CAR T and bispecifics. So what is CAR T?
So T cells are a type of white blood cells, a type of lymphocyte we all have in our body. And you’ve probably seen the white blood cell called lymphocytes in your blood tests. T cells are one of them and they’re pretty amazing.
Remember I told you I had gotten a C in immunology in med school. So I learned a lot during residency and fellowship to do this. But T cells are sort of our army men, right? I think of them as troops, as soldiers, and they have been born with a different receptor. I call that night vision.
So when you have these different T cells that are supposed to fight fungus and viruses and all these things in the world that can harm us, those T cells, they have a specific lock and key with those different pathogens.
What we can do now is we can collect those T cells by blood, almost like a dialysis session. It’s a one time, you know, two to three hour session where we put a line in, take your blood out, put the rest of the blood in, take your T cells out of there, and then we can put a new receptor in there, this new night vision.
So it’s on BCMA, that B cell maturation antigen again. That goes into the cell, through something called a viral vector. Now every T cell that we grow in the lab specifically is made for BCMA. That’s grown and there’s all these other things that help the T cell when it finds that myeloma, it helps it grow better and kill better.
All these things like CD8 linkers and 4-1BB, et cetera, it’s a lot more detailed than you need to know. But really, it’s where we’re giving your T cells something to go find that myeloma. So the two that have been talked about the most are ide-cel and cilta-cel.
Ide-cel is the one that’s just recently approved as standard of care. And there’s a couple of differences between it. But really, I’ll just I’ll show it here. The number of cells you need is a little bit different because of the way the CAR T is made.
But for ide-cel, we usually try to get to 450 million cells and cilta-cel is about 50 million cells because it’s per kilogram so it’s just dosed a little bit differently. But both of the big trials for this had patients who had six lines of therapy. So they had pretty much everything already that was available to them.
When the myeloma becomes refractory to treatments
Dr. Krina Patel: When we say triple class refractory or penta-class refractory, this means the different classes of myeloma drugs. So triple classes, proteasome inhibitors, IMiDs and CD-38 antibodies. And then penta-refractory is that you’ve had all the proteasome inhibitors, two of them, plus the two IMiDs, plus the CD-38 antibody.
These patients were refractory, meaning that they had already relapsed while on these therapies so they had pretty bad disease. And then for the ide-cel, you actually had extra medullary disease, meaning that it’s not just in the bone marrow or in the bone, but you can have it in soft tissue and that disease is a little bit harder to treat. It’s more aggressive.
A lot of these patients had pretty aggressive disease and a lot of them had a lot of myeloma going into it. So you really can’t compare the two in terms of outcomes because the patients are different. But just to show that both had really relapsed refractory patients that had a good amount of disease and high risk disease, too, and those are patients that usually don’t do well on clinical trials or even a single agent therapy.
Here, you see this response rate of 73 percent, 98 percent. I mean, mostly when patients were with daratumumab or BLENREP or selinexor, all these single agents, the response rate is 30 percent when they get approved a single agent. This blew that out of the water right?
This is why I am so excited about this. And I have conflict because I do research with CAR T. But this was huge. This was a great response rate in a patient population that had already had those other drugs.
And, you know, really, we didn’t know what else we would be able to give them. And the deep responses, the CR responses, a third of patients in ide-cel and 80 percent in cilta-cel had these really, really good responses where we couldn’t find the myeloma in the bone marrow anymore.
And same thing with that minimal residual disease, looking at it to the millions and we still couldn’t find those myeloma cells. And then PFS is what I call the hibernation period for myeloma. And if you had that deep response, it was 19 months for ide-cel and in cilta-cel, it looks like it’s 22.8 months for all patients, so pretty impressive.
One time cell dose, no other therapy afterwards. So you’re really just coming in for blood count checks, things like that. And to have that long one-and-half-years to two years without needing any other therapy, that’s a huge window that tells us so much about new things that we’ve learned with myeloma, so I think that’s where the excitement really comes from.
Older patients have had great responses to CAR T
I’m not going to go too much into it. But it just showed that people who are older actually had great response rates.
So they had great response rates. People who had high risk disease still had a really good response rates.
People had extra medullary disease in these triple refractory, penta-refractory. So even our high risk patients had some fantastic responses, which is exciting. And then the other thing for both ide-cel on the next slide for cilta-cel really the patients who had those deepest responses, the CR or stringent CR, which is this light blue line, did way better. That did a lot better for longer than patients who got a VGPR or PR.
So from all of our therapies, we know that the deeper response you get, the better you do. So now our goal is how do we make these patients that are down here come up here? So how can we improve the current therapy to make sure everybody gets that stringent CR? And then how do we hopefully cure some of our patients?
And even with the cilta-cel, the patients who got that stringent CR on the teal line, the green Line did better for longer than the folks who got a little bit less than stringent CR. So that deeper response really matters.
Common CAR T side effects
Dr. Krina Patel: So then quickly into the toxicity, because this is also a completely different toxicity than we have for anything in myeloma. I think most people try to think of this and compare it to stem cell transplant, but it’s not quite the same.
It’s a cell therapy yes, but in transplant, you’re using chemo, you’re using that high dose, melphalan to really just take everything out of the bone marrow and you get the stem cells back for normal immune cells again and platelets.
Here, we’re using those T cells to kill that myeloma. So what happens, it’s like when people have the flu, right? Remember, I told you the T cells are used to killing viruses and fungus, things like that. It usually gives us fevers, sweats. It can give us chills. It can make our blood pressure go up or down.
And that’s really what cytokine release is, that when these T cells engage and they kill that myeloma cell, they release all these cytokines that help other white blood cells come there to help with the fight. And really those cytokines then lead to fevers and just not feeling well, fatigue, etc.
Mostly Grade 1 or 2 cytokine release syndrome (CRS)
Even though there’s a lot of it, 84 percent and 95 percent of patients that get CRS (cytokine release syndrome), these are all Grade 1/2, which is low grade. And so usually we give Tylenol or acetaminophen and that’s it.
We just make sure it’s better and then you’re done and we keep an eye on it. Sometimes you might need a little bit of oxygen or you might need a little bit of fluids. But really, that’s what Grade 1/2 is.
Grade 3 cytokine release syndrome (CRS)
When that small number of patients that get Grade 3 or higher, that’s when we end up saying, OK, we need to watch a little bit closely. Do we have to give medications to help your blood pressure come up?
And really, the nice thing with myeloma patients is that when we give something called tocilizumab. It’s a drug we use for autoimmune disease. It actually helps knock these cytokines down. And people do a lot, lot better and they’re kind of back to normal again.
So for our patients at MD Anderson, we’ve actually had two patients in total go to the ICU and within 12 hours come right back out of the ICU because we get the toci and they’re fine. So it’s just going to a center that knows how to deal with this and they can act on it quickly. But the majority of patients do really well. In most people the differences here are when that CRS happens.
So in ide-cel, they have a lot more cells going in at first. So usually happens at day one versus in cilta-cel they have a smaller number of cells, which takes them longer to get to that point. So usually it happens around seven days.
And then the other ones, obviously your counts can be low. So platelets, white count, neutropenia, you know, we talked about the anemia and we do have to transfuse patients sometimes. Usually after a month or two, most people are back to where they’re okay not needing any of that, but a few patients we have to watch for longer and potentially give more support for a few months and then, of course, ICANS, which is the neurotoxicity.
Thankfully, in myeloma we don’t see it like we do in lymphoma CAR T. But this can potentially lead to some confusion or delirium, as we call it. And if it gets really bad, so Grade 3/4 is when people can potentially have seizures or go into a coma and we don’t really see that, which is good.
We quickly give steroids to knock it down and that usually reverses it completely. So for us, we have patients write a sentence every day because if that sentence changes, that’s an early indication something is happening. So we intervene so it doesn’t get to that Grade 3/4.
Intervening quickly for more serious CRS cases
Dr. Krina Patel: We’ve learned a lot about how to intervene quickly to make sure we don’t get to the really bad levels of where it can. And so with that, I think this is my favorite slide, because this really shows, you know, for my patients when we talk about patient experience and why people are so into CAR T, that one dose of cells.
You do get three days of chemo just to knock your immune system down so that when these cells come in, your immune system doesn’t just clear it all out. We need a little bit of time for those cells to find the myeloma, do what they do, and then your cell. Come up and kind of kill them off.
So this is one of my patients is a high school teacher and one of my patients who a nursing supervisor and both of them were working through covid at school and all this, and their myeloma just took a turn for the worse.
Both of them were able to get onto a CAR T trial and actually are both doing fantastically. They’ve both gone back to work despite COVID, et cetera, and are actually doing really, really well.
So for my patients, what they tell me again and again, can I go on another CAR T trial? Because it gave me that chemo free period. I felt so great I was able to do the things I want to do and hearing that, I can’t not hear that.
This is why I think for me, it’s such a big deal to be able to do something like this. So we’re working on getting more patients standard of care CAR T now, which is a whole nother ball game. And there have been some bumpy roads along the way, but with potentially cilta-cel, hopefully becoming approved also at the end of this year, their FDA approval date is the end of November. I’m hoping by January we will be able to get more and more patients on.
Bispecific T-cell engagers (bispecifics)
Dr. Krina Patel: Going into the bispecifics and really the picture on the side just shows you that there’s two ways bispecifics, there’s multiple ways bispecifics work. But there’s the bispecific antibody, meaning that you have this antibody that attracts the T cell by something called CD3.
It kind of brings that over. And then on the BCMA, it brings the myeloma cell over. I call this the handcuffs right. It’s handcuffing both of them, bringing them next to each other so that T cell can kill that myeloma. So this is sort of an indirect way of getting that T cell there.
Then you have a T cell engager, which is more like magnets. It actually puts both them together and puts a little fire under that T cell and kills that myeloma cells. So, you know, there’s been multiple studies that have been looked at and I’m just showing the response rates of the three that were presented at our big oncology conference, ASCO in June.
Elranatamab overall response rate, 70 percent. Teclistamab overall response rate 65 percent. So these are also still twice to three times what it has been for most other drugs. And those two are both against BCMA. But the other really cool thing is that we’re starting to find new flags or antigens for myeloma and GPRC5D is just one of them.
But we have multiple other ones, too. And so one of those bispecifics was actually talquetamab against this new antigen. And once again, overall response rate, 70 percent.
These are fantastic for single agent.
I’m not going to read this, but you’ll get these. But these are some of the highest overall response rates we’ve ever seen for relapsed refractory myeloma patients. And really, our question for the next 5 to 10 years is how do we sequence, as we talked about with BCMA specifically, or how do we combine autologous transplant BCMA CAR T and or bispecifics, can we give CAR T’s and then bispecifics later to help them work better? Maybe. There’s lots of things that we are wanting to do and hopefully we’ll have trials for soon.
Talking about a myeloma cure
Dr. Krina Patel: Then of course, how do we cure patients with CAR T? If we can get these responses like this in very relapsed refractory patients, how can we finally cure some of our patients?
In lymphoma we can do it, in leukemia, we can do it. So now we’ve got to get smarter about how we can do it for myeloma patients.
That’s really what looking at these mechanisms of response and resistance to help us get smarter about how to fix that bone marrow for once and for all. And so the other patients that really we talked a little bit about the unmet need, those who don’t have access to appropriate health care, clinical trials, we really need to do better here and make sure that all patients get access to care, high disease, plasma cell leukemia.
They do CAR T and bispecifics seemed to work better for them. But if their disease is so bad, they can’t even get to the trial, well, we’ve lost those patients, so we need to figure out a way to get patients newer agents like this earlier.
Relapsed refractory disease for patients who are really aggressive, kidney failure we talked about there are people with CNS or involvement of their brain or the fluid in there around their brain and spine, which is hard to treat.
Then, of course, frail patients with increased comorbidities. And these are the kinds of things that we really need to work on and hopefully will eventually be able to treat all patients and hopefully cure, like I said. And then just a quick plug on the different trials we have at M.D. Anderson for CAR T and bispecifics.
Autologous CAR T we have to collect your own cells as a bridging period, about three to four weeks. And it takes a little bit of time. But most of these trials are for patients who are relapsed refractory, at least three lines of therapy. But as you see up here, we’ve actually been doing trials in earlier lines to see if that works better. And right now, KarMMa 3 has already just finished recently so hopefully we’ll have data on that soon. KarMMa 2 has finished enrollment for the most part, and KarMMa 4 is going to look at up [01:04:00] front patients.
So patients who are high risk that we actually give CAR T to instead of stem cell transplant. So I’m really excited about that trial as well. And then KarMMa 7, we’re actually combining things. So as we said, we’re trying to become smarter about it.
We’re combining different therapies with CAR T-like CELMoDs that we didn’t really talk about, but that could potentially improve CAR T or ways to make that BCMA work better.
Off-the-shelf options
That’s a really exciting trial. And then we have, of course, off the shelf. So people who can’t wait for those four weeks to make their own cells because we have that as a problem, we have allogeneic (allo) CAR T trials where you’re using someone else’s T cells that might be healthier because they haven’t seen cancer or drugs.
There are different trials we have for that, including one that’s against CS1, which is a different antigen. And then we have, of course, our T cell engagers that are BCMA mostly right now, but we have others that are coming down the line for other antigens.
So with that, I just want to thank our patients, caregivers and research teams because we have so many therapies because of all these trials right. And because of our patients and our caregivers who actually volunteer to go on these trials.
Seven years ago when I was a fellow, we didn’t have CD-38 monoclonal antibodies and all these things really. We had novel therapies like bortezomib and lenalidomide, but all these other things were in trial still. And so at this point, we use them like water for myeloma patients because they work so well. And so really it’s because of them that we’ve even gotten to this point where we have options to individualize treatment.
Dr. Muhamed Baljevic: So in the near future, we’ll have sort of four separate, similar, different, but in the same targeting to BCMA for example. So which one do you use? Which one do you pick? It’s a really difficult question to answer.
In short, there is no clear answer right now. We’re going to need studies and trials to help us understand what’s the sequencing going to be like. And as was pointed out by Dr Patel, different trials are going to be asking different questions, including as many were hopeful.

Whether parties can really substitute autologous stem cell transplant to multiple myeloma, right, we’re both transplant physicians at this point, I would say we don’t have enough data yet, but certainly that’s been the hope.
I think that we’re looking at a future where BCMA therapies are going to be brought in earlier in earlier, and we will, after even just two lines of therapy, potentially have for four or five different classes of refractoriness, which really is going to put us at a decision point of what is the best next thing to give.
And that’s why agents like selinexor or melflufen or these other ones and anything else that we will come up with hopefully are so important. That’s not to say that they’re only reserved for those time points.
But the point is, the more we have, the better.
And that the more diverse the mechanisms get, the more hope we have that these resistant mechanisms that Dr. Patel mentioned can be circumvented. So I’ll probably stop there. There will be many other things to talk about, but these are very exciting times for my myeloma.
The Patient Story: Thank you for that. I appreciate that, Dr. Baljevic and Dr. Patel. Any last words from you as well? So the takeaways for patients and caregivers?
Dr. Krina Patel: I think that it’s a good thing that we have so many therapies, a lot of people get overwhelmed and the like, how does anyone pick and are they going to pick the right thing for me? But I think the more options you have, yes, you have to go to someone that knows myeloma or at least help them help your doctor with your treatment.
The importance of seeing a myeloma specialist
But that’s who can actually help to make sure that you get that best therapy. It’s not an algorithm. And I think this is what myeloma has been for the last decade or two, is that when we get new therapies in the relapsed refractory patients who are phase one, phase two trials, usually the Phase Three trials are earlier and earlier.
Then that tells us we can use it sooner. And in the end, it’s still about using your best therapies up front because that usually gives you that longest hibernation period and hopefully one day cure. Right. If we can cure it, we’re hoping, hoping we can cure it at the smoldering stage for patients right before they ever get any kind of organ involvement.
So that’s sort of why it’s set up the way it is. But I think that I wouldn’t be afraid that by using a quadruplet or doing BCMA early that you’re going to run out of options either.
That’s not the case with the goal here is how can we give you chemo free period or how do we reduce therapy? So the likelihood is that even if we give you a quadruplet plus a BCMA, once you get to that best response, we’re going to take things off just like we do now.
The goal is that you actually get a much longer time in that hibernation because of all these things and that more people get a better response. Really, it’s to make sure that everybody gets that response as to why we change things around. So it’s yes, I don’t think I’ll ever be able to retire.
I was hoping to retire in like five years, but I don’t think that’s happening. But I do think that this is huge and why we’re getting so many more people interested in myeloma as physicians and researchers, because that puzzle, we’re sort of putting it together.
And sometimes the more you dug, the more you learn and it becomes a little bit more confusing at times. But once again, it gives us more opportunity to actually treat all patients with myeloma. So it’s really, really exciting. And I think the talk today was fantastic with Muhamad, my co fellow from before. And I’m so happy to be able to do this today with both of you.
The Patient Story: Thank you both so much for spending so much time with us in setting the landscape. I know there was a lot to get through and I think thank you for not retiring yet, Dr Patel. And I hope you, too, Dr. Baljevic.
You know, having people like you who not only clearly know what you’re doing in the research on the research side, but really care about the patients, because I’ve heard it come up several times throughout the entire discussion about the patient experience, about the impact.
Dr. Baljevic talking about financials or financial toxicity. I mean, these are so important. And so we appreciate from patients and caregivers when doctors are holistic in their approach. So thank you so much. And for our audience questions not answered today, we will try and do a follow up. And yes, Dr. Baljevic?
Dr. Muhamed Baljevic: We have limited time in clinics with our patients, and it’s just sometimes not enough to speak about everything that we think is important.
And I know and I’m sure your viewers will agree, as we all feel in this field, that these types of education efforts and the materials that are available to patients that stimulate them to think about their disease, that stimulate them to think about reaching out to specialists at least once during their disease course, really important because they are pushing the boundaries forward and forward.
We know that there’s data that clearly talks about improved outcomes when we all come together. And I just wanted to use this opportunity to thank you for that work. And it was a pleasure to speak with both of you today.
The Patient Story: Thank you so much, I feel very grateful to do this work, and as we know, a lot of people in this country don’t have access to larger health care providers and academic centers and to people like you.
So we’re going to push this out as much as possible so we can reach as many people as possible. And for the full talk and for both stories, we will post it at ThePatientStory.com, where you’ll find human answers to your cancer questions. Thank you so much.


Than you Dr. Patel & Dr. Baljevic!
More from Myeloma Specialists
No shortCode found
Relapsed/Refractory Multiple Myeloma Patient Stories
Dr. Yvonne D., Relapsed/Refractory Multiple Myeloma
Symptoms: Severe hip pain, trouble walking due to a broken pelvis, extreme fatigue, bone pains
Treatments: Chemotherapy, stem cell transplant, radiation therapy, surgeries, CAR T-cell therapy
Michele J., Relapsed/Refractory Multiple Myeloma
Symptoms: Fatigue, anemia, persistent lower back pain, sharp leg pain during movement
Treatments: Surgery, chemotherapy, stem cell transplant
Theresa T., Relapsed/Refractory Multiple Myeloma, IgG kappa Light Chain
Symptom: Extreme pain in right hip
Treatments: Chemotherapy, CAR T-cell therapy, stem cell transplant, radiation
Laura E., Multiple Myeloma, IgG kappa
Symptom: Increasing back pain
Treatments: Chemotherapy, stem cell transplant, bispecific antibodies
Donna K., Refractory Multiple Myeloma
Symptom: None; found through blood tests
Treatments: Total Therapy Four, carfilzomib + pomalidomide, daratumumab + lenalidomide, CAR T-cell therapy, selinexor-carfilzomib





