Bladder Cancer Breakthroughs 2025
New Treatments & Bladder-Sparing Advances
Bladder cancer care is evolving rapidly, with new treatments offering the potential for longer survival and better quality of life—including the possibility of preserving the bladder.
In this expert-led discussion, MD Anderson’s Dr. Ashish Kamat breaks down the latest in research, emerging therapies, and what patients and care partners need to know now to make informed treatment decisions.

We would like to thank our promotional parter, The World Bladder Cancer Patient Coalition (WBCPC), which brings together bladder cancer patient organisations from around the globe to improve the lives of people affected by bladder cancer. They are committed to raising awareness, providing trusted patient information, and ensuring the patient voice is heard in research, policy, and care.
Edited by: Katrina Villareal
- Introduction
- What Inspires Dr. Kamat to Work with Bladder Cancer Patients?
- Categories of Bladder Cancer Patients
- How Common are the Different Types of Bladder Cancer?
- Getting Patients and Care Partners Involved in Treatment Decisions
- How Important are Biomarkers in Making Treatment Decisions?
- Treatment for Low-Grade vs. High-Grade Bladder Cancer
- The Role of Biomarkers in Muscle Invasive Bladder Cancer
- Your Bladder Cancer Team: Who’s Involved?
- Concerns from Patients About Their Bladder Cancer Treatment
- How to Find the Right Provider
- Exciting New Cancer Treatments on the Horizon
- Current Clinical Trials
- Are New Bladder Cancer Treatments Decreasing Side Effects?
- Understanding TAR-200 (“Pretzel” Device)
- Understanding Treatment with EV + Pembrolizumab (EV+P)
- Humanizing Clinical Trials
- Conclusion
Introduction
Stephanie Chuang: My name is Stephanie Chuang. I’m a cancer survivor, patient advocate, and founder of The Patient Story. The mission came from my experience of trying to deal with a diagnosis and navigating through all of the questions and not knowing.
Here at The Patient Story, we try to humanize this information for you and part of that is to provide access to topic-specific cancer experts, such as Dr. Ashish Kamat from MD Anderson, so that you can learn more and empower yourself or your loved one in your care. We’ve had more than 100 million views of our in-depth story videos that mostly feature patients, sometimes caregivers, care partners, and doctors. Our goal is to help promote self-advocacy and connection.
While we hope this is helpful, this is not a substitute for medical advice. Please consult with your healthcare team when making treatment decisions.



We’re talking about bladder cancer treatments in 2025 and beyond. This follows big meetings, including the 2025 American Urological Association (AUA) annual meeting, where top researchers gather to learn and discuss the very latest. Our focus is on what’s new and promising, all through the lens of what patients and care partners need and want to know about to stay informed and empowered.
Leading this important conversation is Dr. Ashish Kamat, an endowed professor of urologic oncology at MD Anderson Cancer Center in Texas. He leads many patient advocacy groups and efforts, including the World Bladder Cancer Coalition. He’s a global leader in bladder cancer research, clinical trials, and bladder-sparing strategies. He’s been in the field for over 20 years and has published a few hundred publications. He’s the president of the International Bladder Cancer Group (IBCG) and the International Bladder Cancer Network (IBCN).
Dr. Kamat, I already told you, I’ve got to pause after that. You’ve got so many things and accolades, but what I want to say is thank you for joining us.
What Inspires Dr. Kamat to Work with Bladder Cancer Patients?
Stephanie: I’ve seen your name in so many patient-facing efforts and discussions and I’d love to ask you. What drives you to go beyond the clinic, to try and get as much information and awareness to as many patients and care partners as possible, even those who are not in your direct care?
Dr. Ashish Kamat: First, thank you so much for having me. As you said, it’s very important to reach out to patients, not just because that is what we do, but if we don’t do that and we don’t get patients and their carers to be educated, involved, and understand the disease process, then we can’t give them the best chance of a cure. It’s not just surgery, medication, or radiation, but it’s a true partnership. In some ways, it’s a selfish motive to get patients more educated because it helps me give them the best results, which in turn helps both of us.
Stephanie: I love that. In the spirit of this conversation, I read one of your quotes, which was, “Nothing is more rewarding than hearing a patient say, ‘We made the decision together,’” and I loved reading that. Thank you so much for all that you do.
Categories of Bladder Cancer Patients
Stephanie: Let’s start this conversation by addressing some of the basics of bladder cancer for those who are newer to the diagnosis. Of course, this conversation is not individualized, so we’re not able to address every single potential situation on diagnosis. But in broad strokes, how do you categorize patients in determining what treatment options you might recommend? Would that be muscle invasive or not, high- or low-grade, and staging? What goes into your ultimate recommendations when you’re talking to patients?
Dr. Kamat: The first thing I always want a patient to understand is what disease state they have. From that perspective, I always try to have the patient think of their bladder as a balloon and the muscle layer as the rubber of the balloon. Just like a balloon, when the bladder’s empty, it’s collapsed and crumpled. When it fills up with urine, it gets larger and thinner.
It’s important for the patient to understand that just because it’s not muscle invasive, if it’s high-grade, it can still be a threat to life.
Dr. Ashish Kamat
With that analogy, think of the muscle layer being the rubber of the balloon — the two layers on the inside and the muscle layer on the outside. When these tumors start, they start on the inner surface of the bladder, but they set their roots down towards the muscle of the bladder. That muscle of the bladder getting involved — or the rubber of the balloon, so to speak — is the key determining step as to whether it’s safe to save the bladder or not. That’s the first thing that I like patients to understand.
Do you have muscle invasive disease, where our focus shifts to whether we can save the bladder and, if not, do we need to do radical surgery? Do we need chemotherapy? Do we need to do trimodal therapy with radiation? Or is the tumor not even in the muscle yet? In that case, we’ll aim to save the bladder, but then what do we need to do?
Once I go through that broad categorization, then I help the patient understand: is it a low-grade disease or a high-grade disease? When the tumor is noninvasive, you can have a low-grade disease, which again is still cancer, but for most practical purposes, you can almost treat them like warts. They’re nuisance factors and require intervention, but they’re not going to threaten our patients’ lives.
On the other hand, you have high-grade tumors, which may be small — smaller than just a point of a pencil — but they can kill the patient. It’s important for the patient to understand that just because it’s not muscle invasive, if it’s high-grade, it can still be a threat to life. That’s the majority of patients that we see.
Of course, there is a small percentage of patients who present with novel metastatic disease, where the cancer’s already spread outside the bladder to other parts of the body. When that happens, I try to help the patient focus our discussion. If the tumor’s left the bladder, we focus on how we have to treat the rest of the body first and then bring our attention to the bladder to see if, at some point, we still need to address what happened in the bladder to begin with.
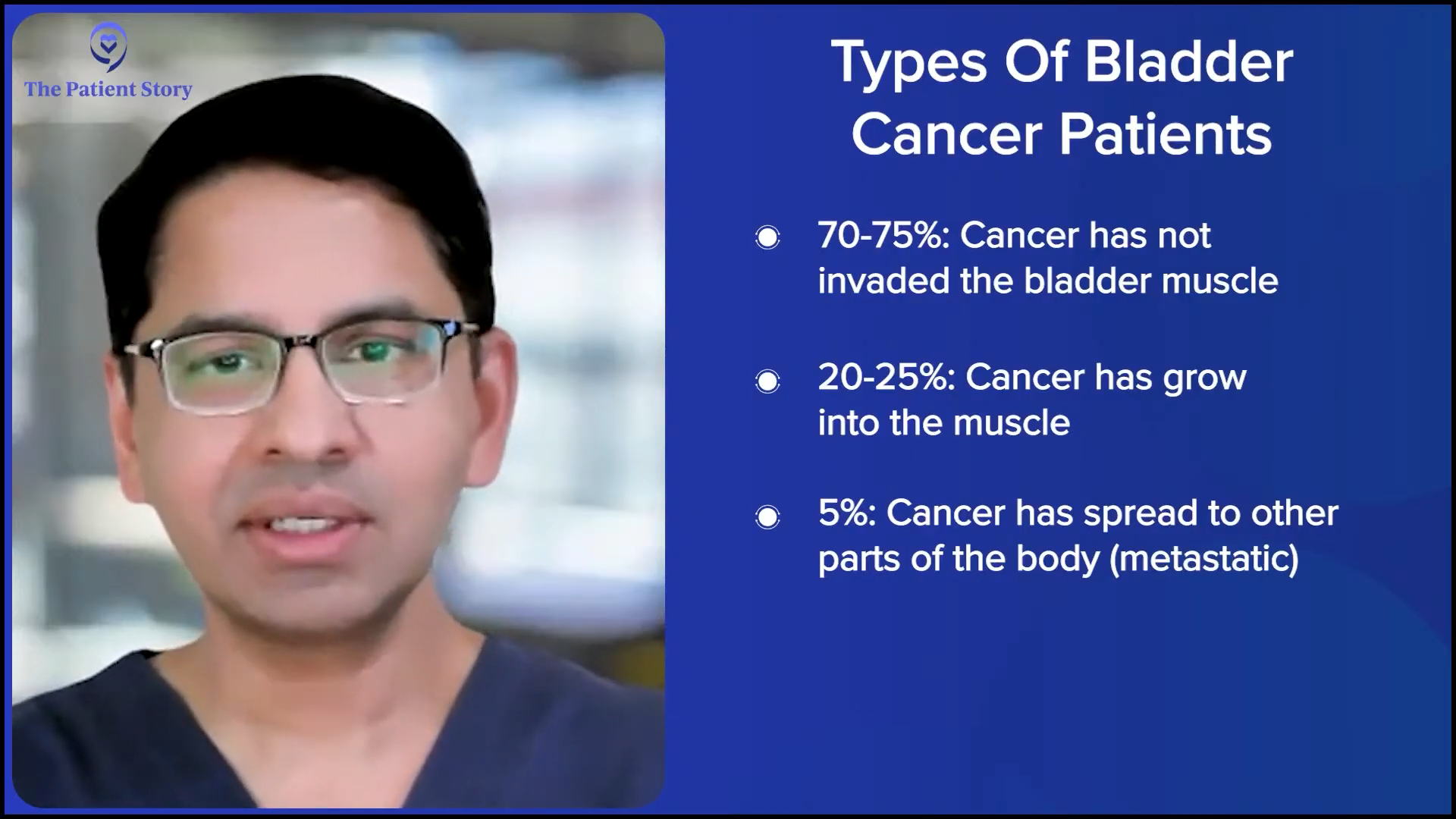
How Common are the Different Types of Bladder Cancer?
Stephanie: How many patients typically land in these categories?
Dr. Kamat: The most common presentation for a patient is non-muscle invasive disease. Usually, about 70 to 75% of patients will present with noninvasive disease. Roughly 20 to 25% patients will present with muscle invasive disease, where it’s already in the muscle of the bladder. Fortunately, only about 5% of patients present with metastatic disease because that’s where a cure is hard.
If you see blood in the urine, don’t ignore it. Let someone know.
Dr. Ashish Kamat
Unfortunately, it is gender-differentiated. Women tend to have a higher risk or percentage of presenting with more advanced stages of disease. Partly because when the most common presenting symptom of bladder cancer is blood in the urine, you can imagine many women, especially young women, get told, “It’s not anything to worry about. It’s probably that time of the month. It’s probably a contaminated sample.” It’s important for primary care physicians and females to know that if you see blood in the urine, don’t ignore it. Let someone know.
Stephanie: I appreciate that. We’ve certainly done lots of these stories and that’s something we hear commonly. Especially as a woman, it’s easy to dismiss or attribute it to something else. Always, if you don’t feel well, if something’s not right, or if something’s concerning, you’re saying to advocate for yourself. Find someone who’s going to address that. Is that right?
Dr. Kamat: Absolutely. The best advocate a patient can have is themselves and their family, and, of course, us as partners, but they have to find the right advocate. Before they find someone to advocate for them, I want patients to empower themselves to say, “I’m not willing to take this response that you’re giving me, saying it’s nothing to worry about. I hope it’s nothing to worry about, but tell me why it’s nothing to worry about.”

Getting Patients and Care Partners Involved in Treatment Decisions
Stephanie: I also read that non-muscle invasive bladder cancer, which, as you said, most people are diagnosed with, behaves more like a chronic condition that requires ongoing care, but it does require that because of high recurrence rates. Can you talk more about how that informs your approach with patients?
Dr. Kamat: Stephanie, that’s where it’s important to get the patient and their family involved early to set expectations a little bit. But also, I like to ask patients, “Tell me what’s important to you.” Non-muscle invasive bladder cancer is a chronic condition and we want to make it a chronic condition. We don’t want to make it such that a patient is diagnosed and then they die from their cancer. If you do the right thing and we treat the patients correctly, we are saving their bladder, but then the bladder is at risk of the tumor recurring.
In some ways, I allude to this with patients to make it easy to understand. Think of your backyard. You have a couple of weeds. You see them and you want to treat them, but then you still have the backyard in place, right? And unless you remove the entire sod, you’re going to have weeds pop up.

Some patients will say, “You know what? I can’t keep coming to the doctor’s office every three to six months. I live too far away. I had to have my grandson or granddaughter take time off to bring me there. Can you give me one-and-done treatment?” In that situation, sometimes even for a noninvasive disease, we will do a radical cystectomy, which is to remove the entire bladder. Yes, that is a drastic step, but for particular patients — unfortunately, a small number of patients — that is something that they want because it’s one and done.
I don’t encourage that, but since you asked, it’s important for me to know from a patient their desire for their treatment paradigm. If a patient tells me that they want a one-shot treatment, then we have to take a drastic step. But for most patients, it involves regular checkups, regular treatments, evaluation of the bladder, cystoscopy, cytology, imaging, etc.
How Important are Biomarkers in Making Treatment Decisions?
Stephanie: For a lot of people, their decision-making is largely based on efficacy, like how well this will treat the disease, but also the impact on quality of life, which is something we talk about across all kinds of cancers. Is there anything else upfront that you want to make sure that patients and their families understand? Are there other factors, for instance, that might inform a different way of treating them? We hear a lot about biomarkers in the space of solid tumors, in particular.
Dr. Kamat: Biomarkers is a field where we and most of us in this oncology space are very interested in. They help us understand the biology and differentiation of bladder cancer treatments, paradigms, and development, etc. But in some ways, we’re fortunate in the sense that the best treatment for non-muscle invasive bladder cancer is biomarker-agnostic. It’s an immune therapy called intravesical BCG, which works so well that we haven’t needed to use biomarkers to change or inform the treatment paradigm.
Don’t spend your own money doing all of this. It’s not going to help you or us upfront.
Dr. Ashish Kamat
Sometimes I see patients walk in with sheets of paper where they’ve paid out of pocket to get all this testing done. It’s not helpful, other than as a research or educational tool. If a patient wants to understand, “Is my cancer being driven by FGFR3, PD-1, or something else?” That’s great for education, but it doesn’t help us in today’s day and age with the upfront treatment.
Now, if the tumor doesn’t respond to first-line therapy, then we will want to do biomarker analyses because there are FGFR-directed therapies and specific Rb-directed therapies. But for that, we want to look at the tumor that develops after the treatment hasn’t worked. Looking at the first tumor itself is not cost-effective for the patient. From a research perspective, we do biomarker analyses all the time. This is more for patients. Don’t spend your own money doing all of this. It’s not going to help you or us upfront.
Stephanie: Thank you. That’s important because we hear things in the world of science and major news publications, and it’s hard to understand what applies to our specific situation and cancer.
Treatment for Low-Grade vs. High-Grade Bladder Cancer
Stephanie: Before we dive into more of the latest and promising therapies and options, what typically is the treatment decision-making path or the options given based on these larger categories of patients? If you’re talking about the non-muscle invasive bladder cancer and then high-grade versus low-grade, here’s how we usually do this for low-grade and here’s what we normally recommend for high-grade. Could you provide a more basic picture of what that landscape is?
Dr. Kamat: So let’s assume a patient is sitting in front of me and he or she has low-grade, non-muscle invasive bladder cancer. The first thing is to explain to the patient that that’s great news. If you’re going to have any kind of bladder cancer, this is the one to have. Yes, it is cancer, but it’s extremely treatable and even though it might recur at some point in your life, the recurrence is not a problem
For the treatment of low-grade, non-muscle invasive bladder cancer, the treatment is removing the tumor, which is usually done with the transurethral resection (TURBT). We look in the bladder with a scope. Currently, we use electricity to remove the tumor. Some places use lasers, but there’s no real benefit to one over the other. Then we give a single shot of chemotherapy into the bladder. That’s what the majority of patients need and what the majority of patients respond to.

Now, some patients have a recurrence of the tumor and as long as the tumor stays low-grade, that patient then transitions into intermediate-risk. It’s not high-risk. The only reason we call it intermediate-risk is because we want people, physicians especially, to understand that it’s gone from low-risk to intermediate-risk, so we might need something more. What is that something more? Intravesical therapy, either in the form of chemotherapy maintenance — so once a week for six weeks and then monthly chemotherapy maintenance — or intravesical BCG.
Now there’s a shortage of BCG across the globe. In today’s day and age, we don’t tend to recommend BCG for patients with low-grade disease, not because it doesn’t work well, but because there’s not enough BCG and it is something we need to reserve for our high-risk patients. For the low-grade, intermediate-risk patient category, the treatment is still intravesical chemotherapy.
Now there are paradigms for patients who are too sick to go to the operating room or who’ve had a recent myocardial infarction (MI) or heart attack and they’re on blood thinners, an antiplatelet drug like clopidogrel (Plavix), or aspirin, and we want to avoid a procedure. We can ablate these tumors with chemotherapy upfront. There are newly-approved agents, like the recently FDA-approved MitoGel (now known as Jelmyto), which is mitomycin in a gel format, where you can ablate the tumor. But that’s, in some ways, a select group of patients who are trying to avoid resection of the tumor or we are trying to buy some time before we can take them to the operating room.

Now, moving from the low-grade patient with low-risk or intermediate-risk category, the next category is the high-risk patient. Any patient with high-grade tumors is considered high-risk, which includes carcinoma in situ (CIS) or T1 disease. There are a lot of factors that come into play with regard to treatment recommendations.
Patients who have high-risk tumors can be further categorized according to their actual risk. If they have certain parameters that make them extremely high-risk — and I’m talking about risk of progression and metastatic disease — I might recommend to a patient, saying, “Your tumor is non-muscle invasive, but it’s getting there. It’s getting to become muscle invasive, so you might still want to consider radical treatment upfront.” Fortunately, that’s a small percentage of patients. For the majority of patients, we can still recommend treatments that allow us to spare their bladders.
The gold standard for many years — for the last 40-plus years and it’s not been dethroned yet — is intravesical immunotherapy with BCG. BCG (Bacillus Calmette-Guérin) is developed from the tuberculosis vaccine. It’s been used for other cancers in the past, like melanoma and leukemia, but it was toxic because you had to give it in the blood. With bladder cancer, we can put it in the bladder.
Early data suggested that it was fairly toxic to patients, which is because people didn’t understand how to use it. If it’s used appropriately with appropriate pre-medication or interval spacing, etc., 90% of patients can finish the whole three-year prescribed course. If you use adequate BCG, the recurrence rates are in the teens and the progression rates are in single digits. An efficacy rate of 90% when it comes to preventing progression of disease of high-grade bladder cancer allows 90% of patients to spare their bladder.

Again, nothing’s 100%. If it doesn’t work or patients can’t tolerate it, we have to use other treatments and that’s where you’ve seen this explosion of drugs coming up recently. There’s gene therapy with nadofaragene firadenovec-vncg (Adstiladrin). There’s cretostimogene grenadenorepvec. There’s an intravesical pretzel device. There is nogapendekin alfa inbakicept (Anktiva), which is the bioshield that Dr. Soon-Shiong has been talking about all over the world recently. All of those come in after BCG has not worked for a particular patient.
Stephanie: Thank you. I know it’s a lot to talk through. We will be talking about all this, but, of course, the explosion, so that people understand what they can consider and weigh might be good for them personally.
The Role of Biomarkers in Muscle Invasive Bladder Cancer
Stephanie: Biomarkers haven’t been a thing yet in bladder cancer. That’s largely a good thing, in that most people don’t require that. The standard of care has worked well. But as you have mentioned, there are meetings and gatherings where people are trying to figure out more about how they may play a role in bladder cancer, is that right?
Dr. Kamat: Absolutely, Stephanie. We were talking about noninvasive disease. If we move into the muscle invasive space, there, the biomarkers are showing more promise and slightly more utility. They’re still not used in the clinic. They still don’t inform us of how we counsel a patient, but they help us inform the patient about their prognosis. For patients who have muscle invasive disease, the discussion becomes, “How can we get you the best chance of long-term cure?” And that usually involves some form of bladder removal.
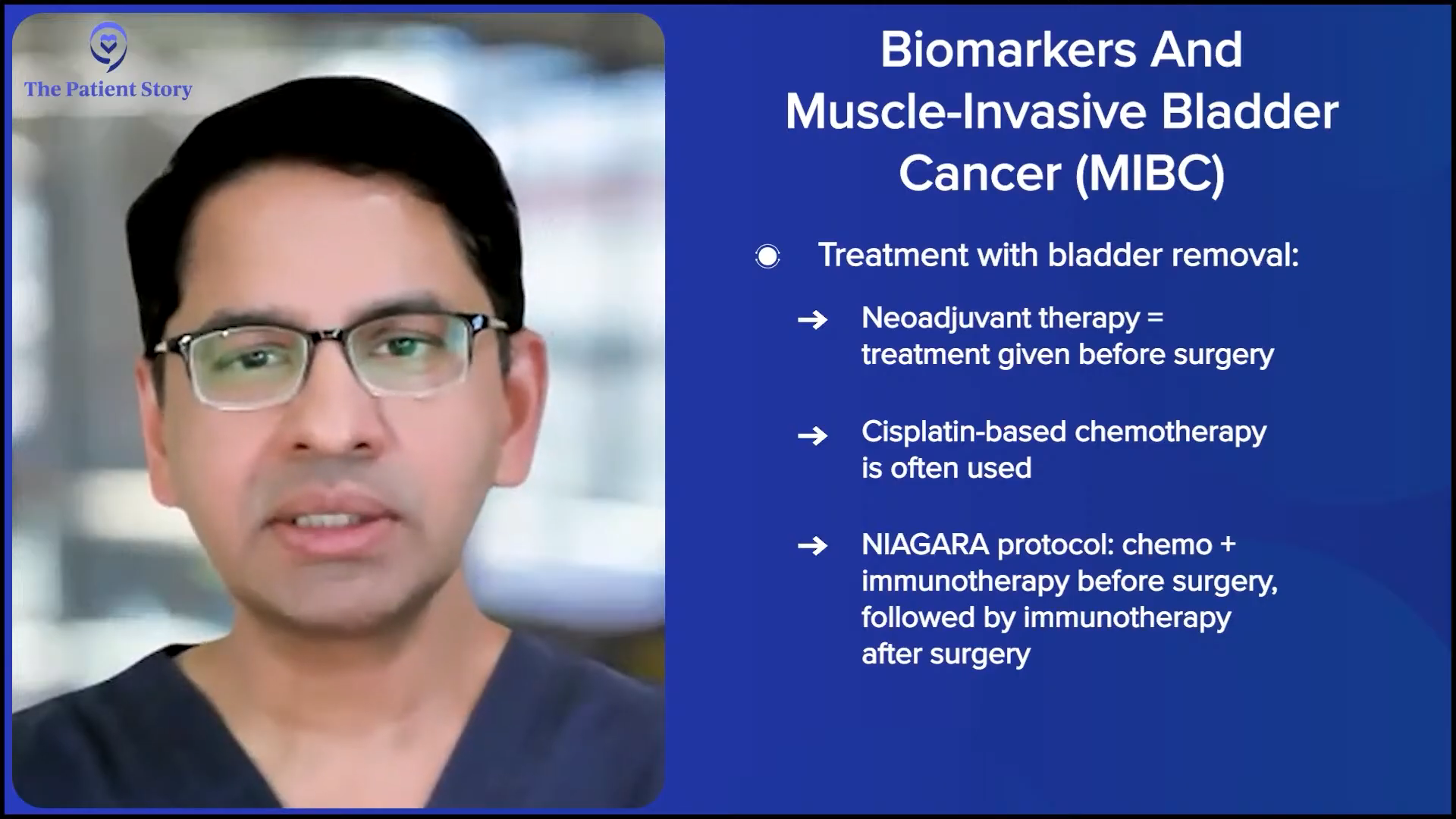
There is trimodal therapy, which, in selected patients, can offer the option of sparing the bladder and providing long-term control, but that’s the minority of patients. The majority of patients do require radical cystectomy, unfortunately, to remove the bladder. And then to give them the best chance of long-term cure, there is neoadjuvant therapy and with the new paradigm, sandwich therapy that comes into play.
Neoadjuvant therapy essentially means getting treatment before the surgery. Traditionally, it involves cisplatin-based chemotherapy, which can be fairly toxic. Roughly 40% of patients can’t even get cisplatin because of cardiac disease, renal dysfunction, age, neuropathy, or something else, so other drugs have been studied and are being studied to see how they can help us give patients who are not as cisplatin candidates some treatment.
In more recent times, the cisplatin combination has been combined with immunotherapy, immune checkpoint inhibitors, and the NIAGARA protocol, which is the most recent development in the space. This protocol essentially suggests that combining combination chemotherapy with a checkpoint inhibitor before surgery and then continuing the checkpoint inhibitor after surgery can offer the patients the best long-term chance of cure in the muscle invasive space.

This is where the biomarkers have come in handy. There are biomarkers that people have developed, trying to help us understand which patients may respond so well to systemic therapy that we don’t have to take the bladder out. Again, this is being studied. It’s not something that I can say a patient should go to their physician and say, “I have this biomarker. I don’t want my bladder out.” No, it’s not ready for primetime, but we’re looking at those. There are DNA repair genes. There are other agents and other markers we’re looking at.
The biggest buzz is around circulating tumor DNA (ctDNA) because data and evidence have come out that suggest that in patients who have detectable levels of circulating tumor DNA, it puts them in a category for prognosis that is not as good. And if they don’t have circulating tumor DNA, then it gives them a better prognosis. Again, this is a research field.
But it’s been studied in the sense that if a patient has elevated ctDNA, do we need to escalate their treatment? If they don’t have circulating tumor DNA, can we de-escalate that treatment? In other words, less toxicity but the same efficacy. A trade-off, of course, is that we need to offer patients shared decision-making because, like I said, it’s currently not standard of care. It still is a research question.
Everything is crucial because bladder cancer is not one of those diseases that you get second chances with, so you want to get the best treatment right up front. The whole team effort is very important.
Dr. Ashish Kamat
Your Bladder Cancer Team: Who’s Involved?
Stephanie: Before we dive even deeper into the details of what’s the latest, typically — and I know it depends on whether someone’s getting care at an MD Anderson versus a community provider or a different practice — what is the makeup of the bladder cancer care team and does that impact what you recommend to people?
Dr. Kamat: For all the time I’ve been doing this and in many parts of the world when I travel and help set up their cancer programs, one of the things I’ve been a champion of is to emphasize that bladder cancer is a multidisciplinary cancer. In order to give our patients the best chance of a cure, it has to be such that everything is considered. Yes, I’m a urologic oncologist on the surgical side, but bladder cancer is not a surgical disease, a medical disease, or a radiation disease. We have to look at the patient holistically.
That’s why whenever we’re guiding patients as to where to get their care, I can’t have everybody flying to Houston to be treated here at MD Anderson, but I tell them, “Wherever you seek your care, make sure that the care team over there is not just one person. That it involves not just the surgeon, but also the medical oncologist, the radiation oncologist, the nursing team, and the support staff.” Everything is crucial because bladder cancer is not one of those diseases that you get second chances with, so you want to get the best treatment right up front. The whole team effort is very important.

Concerns from Patients About Their Bladder Cancer Treatment
Stephanie: I’m sure a lot of people have traveled to see you, given your expertise. Are there common situations you’re hearing from them? Like, “Hey, I saw such and such. They’re not bladder cancer specialist and they wanted to immediately go to this step.” Or maybe things that you’re seeing at different levels that might make it harder for patients to understand what’s good for them?
Dr. Kamat: Yeah, and that’s why organizations like The Patient Story are very important because I see that very often. Patients will come in and say, “This is what I was told. I have no choice. I have to do this.”
First off, every patient has a choice, so when I hear that, it makes me not very happy because patients always have a choice. Our role is to guide them to make what I think is the best choice for them, but it’s very patient-specific. Like I said earlier, what may be right for one patient, like surgery, may not be right for someone else and it might be purely based on their beliefs or their support structure.
Patients always have a choice. Our role is to guide them to make what I think is the best choice for them, but it’s very patient-specific.
Dr. Ashish Kamat
But the other thing that often gets us is that if a patient goes to see someone who doesn’t do bladder cancer all the time, it’s not their fault. It’s not the physician’s fault. The physician is trying to do the best they can, but if they’re treating 10 other cancers or 10 other problems, I can’t expect them to be at the forefront of the latest in that arena. So they’re now offering the patient what is the best treatment in their honest opinion, which might not be the latest cutting-edge.
I always recommend that patients get a second opinion. Even if they come to see me and they want a second opinion from somewhere else, go get it from somewhere else. It’s good for patients to hear, from at least two separate people, if that treatment truly is the best treatment for them and then make the decision.
Now, of course, when patients have to get treated — like with chemotherapy, for example — I often tell them, “You don’t have to be stuck in Houston for the entire duration. Go back home. Your physicians are extremely qualified.” Everybody can give chemotherapy. As long as they’re willing to follow our protocol or our recommendations, get the chemo where it’s easier for the patient.

When it comes to surgery, it’s slightly different because surgery for bladder cancer is extremely specialized; it’s not something that most people can do unless they’re highly trained and it’s not something that I recommend patients go to someone who does it once in a while. It’s very important to go to someone who does it all the time, has a team to support them, not just the surgeon, but the nursing staff, the ICU staff, the anesthesia, the stoma nurses, etc.
Surgery for bladder cancer is extremely specialized; it’s not something that most people can do unless they’re highly trained and it’s not something that I recommend patients go to someone who does it once in a while.
Dr. Ashish Kamat
Ironically, in many ways, people forget that that’s just as important when it comes to radiation therapy. Even in radiation therapy, it’s not the machine that’s necessarily important. There might be multiple places in the country that have a good machine, but it’s the radiation technologist and the radiation oncologist that’s planning the treatment paradigm who can make a difference between radiation that helps save the patient’s bladder versus radiation that causes so many side effects that the patient then loses their bladder and their rectum. That is very important.

How to Find the Right Provider
Stephanie: Where can people go to figure that out? MD Anderson is world-class and people know about it. But in terms of figuring out if someone is the right surgeon or the right institution with people who can do radiation therapy with the precision that we need, how would people go about finding that?
Dr. Kamat: There’s a lot of junk online, but there are some good resources. Your institution, of course. The Bladder Cancer Advocacy Network (BCAN) is a very good, reliable place where patients can go because it’s all for patients and by patients. I recommend that patients make that their first stop. On a global scale, the World Bladder Cancer Patient Coalition has good resources.
It’s the radiation technologist and the radiation oncologist that’s planning the treatment paradigm who can make a difference.
Dr. Ashish Kamat
But I always tell patients to ask their family doctor and local physicians. “Who do you trust here? Who would a patient’s family doctor send their own family to?” Because that’s important as well. Not everybody who does bladder cancer makes it onto these resource sites. Just because I haven’t trained someone or haven’t heard of them, but they’re in Boise, Idaho, doesn’t mean they’re not doing excellent work. The local community of physicians often knows who is good. Conversely, often they know who may have published 5,000 papers, but is horrible. It’s not just academia. It is taking care of a patient that’s important.
Stephanie: I appreciate a lot of what you said. I also want to go back to what you said earlier, which is that it’s not that the practicing physicians aren’t smart; it’s that they are generalists. There’s no way, with all the advancements happening at the pace they’re happening at, that they could keep up with all of the things that are happening in the research. Thank you for that.
Exciting New Cancer Treatments on the Horizon
Stephanie: Let’s dive in more. You already talked about some of the explosion of options that have come up recently. You talked about gene therapy, pretzel devices, and BioShield coming in after BCG hadn’t worked. Can we go through some of the ones that you’re most excited by? And again, in the spirit of what patient group would be most interested in each of these options?
Dr. Kamat: The biggest explosion of data has been in two separate spaces. The first one is for the patient who has high-risk bladder cancer, has tried BCG and it hasn’t worked, and is now faced with a conundrum as to what to do next. The standard treatment for many years was to remove the bladder because there were no real drugs approved and there was nothing available. Unfortunately, when drugs were approved, they had a 4 to 5% success rate at two years. No patient wants to hear, “I’m going to try something with a 4 or 5% success rate.”
Because of that, different organizations came together and the FDA partnered with a lot of us and developed this paradigm of BCG-unresponsive disease. As many patients might know, there was an explosion of drugs studied in that space and currently has the most activity. If a patient has tried BCG and still has recurrent high-grade disease in the bladder, what are the options? That’s where the drugs have been approved, that’s where drugs are being studied, and that’s where some of the agents that I talked about come into play.

The first drug that was approved in that space is pembrolizumab (Keytruda), which is a systemic immuno-oncology (IO) therapy and the results were very exciting at that time, but there were some toxicities. Not many patients today will get single-agent pembrolizumab (Keytruda) for BCG-unresponsive disease, but there are studies using pembrolizumab (Keytruda) with other agents that I think will offer patients the opportunity to get that systemic therapy backbone plus something else.
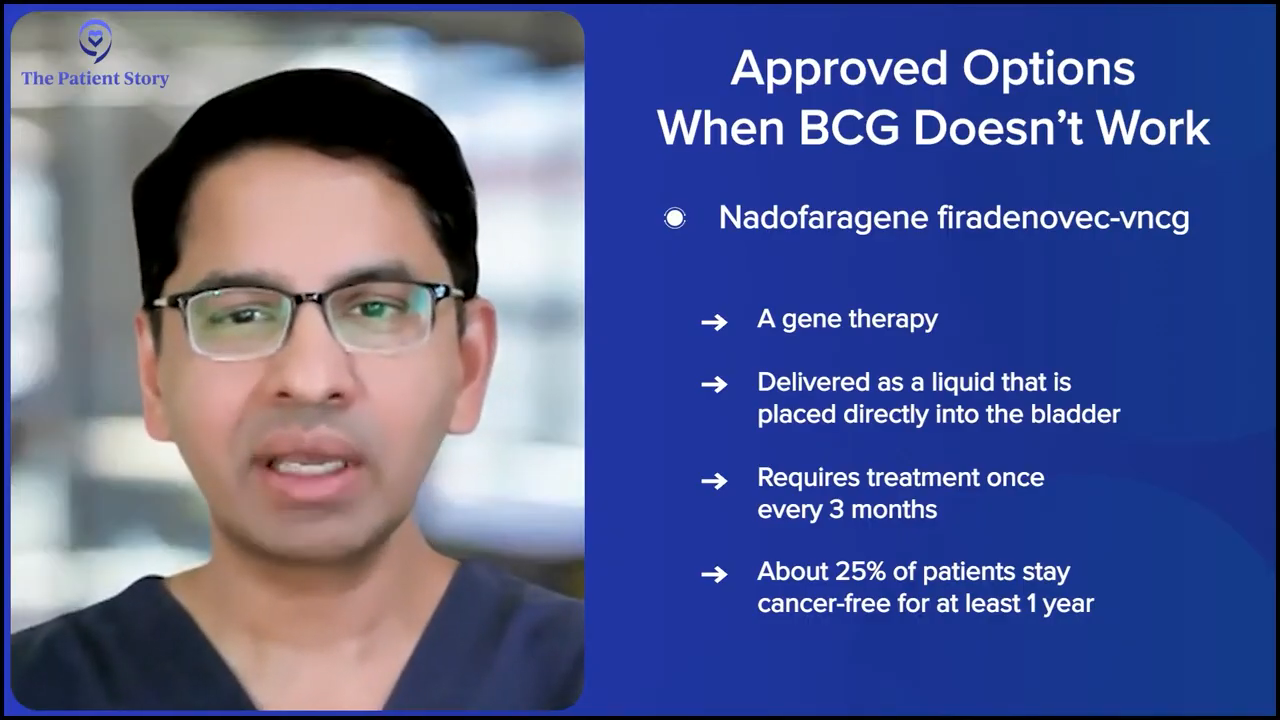
The next agent that was approved in that space was intravesical gene therapy, nadofaragene firadenovec-vncg (Adstiladrin). Now this is a gene therapy that allows the patient’s bladder to, in some ways, become a bioreactor because it transfects the bladder cells with an adenovirus that then helps with the production of local interferon-alfa 2b (IFNα2b).
That has the advantage in the sense that it’s given in the bladder once every three months, so the patient doesn’t have to come to the clinic every week or every other week. It’s once every three months. The efficacy number, roughly at about 12 months, is that 25% of patients will have a positive result and be in disease remission. And that’s great, right? Because we can tell the patients, “It’s approved for BCG-unresponsive CIS. Let’s try it. If it doesn’t work, we’ll know and we can switch to something else. But if it works, it’s great because it’s once every three months and you might have at least a 25% chance of saving your bladder just with this treatment.”

The third drug that was approved in this arena is the IL-15 superagonist, which I’ve seen a lot of buzz about. It’s been called the “BioShield” by some people because there is some evidence that IL-15 might have efficacy across different cancers, but that’s a different topic. For bladder cancer, it’s given in combination with BCG and helps boost the patient’s bladder’s immune response to BCG itself.
That has excellent results in the sense that more than 50% of patients have a response in a year. The downside of that is that it has to be given in combination with BCG. Again, kudos to the company because they have gotten the FDA to agree to allow non-US BCG to be brought into the country and used in combination with this agent, especially in places where there is no BCG.

Those are the approved drugs. The same space now has multiple different drugs that are being studied. One is cretostimogene grenadenorepvec, which is another gene therapy that focuses on the retinoblastoma (Rb) pathway. It’s an oncolytic virus. It has excellent results. It’s not approved yet, but many of us expect that it will be approved.

Then there’s the intravesical pretzel device, which is where chemotherapy — in this case, gemcitabine (Inlexzo) — is put in a little silicone device that looks like a tiny pretzel. The company doesn’t like us calling it the pretzel, but it’s what helps patients understand. It’s put in the bladder and releases the drug at a slow rate. It’s great for patients because you put the device in once and then the patient just has to come in after three weeks or 12 weeks, depending on what stage they’re at, and have the device removed, but it’s constantly releasing gemcitabine (Inlexzo). Again, it’s not approved, but the data is looking promising. Everything that was presented at AUA, American Society of Clinical Oncology (ASCO), and elsewhere looks like this is something that’s going to be beneficial to our patients.

Then there are other agents. There is non-viral gene therapy, such as enGene, which allows patients to get gene therapy without using a virus. Some patients are afraid of the whole viral paradigm when it comes to gene therapy. Some hospitals in smaller communities can’t get certification to use gene therapy that’s viral-based. We don’t know yet, but if it’s approved, it’ll allow patients in smaller centers and smaller communities to get this drug.
Beyond this, I could go on for two hours, but there are so many more drugs being looked at, like laser therapy, etc., in this space. If you’re going to have bladder cancer, this is a great time because there are potentially many options that are going to be available.
Stephanie: I know that we could spend much more time going through every single amazing potential development and promising research happening, but let’s dive into some of the ones that you already talked about and then I have follow-up questions about how you would offer what therapy at what time. In any space, there are questions about whether you use a lot upfront first and try to hit hard in certain diagnoses or not.
First, you talked about gene therapy. Can you say the name of the therapy and share a little bit more about how close we might be? I know you don’t have a crystal ball, but how close are we to seeing this in a clinic? You can’t tell if the FDA is going to approve something, but if it’s promising and it does get approved, in six months, could it be something off of clinical trial, approved, and available?

Dr. Kamat: To clarify, the gene therapy nadofaragene firadenovec-vncg (Adstiladrin) is already approved. That’s interferon-alfa therapy. It’s already approved and patients are using it, and we can actually offer it to patients.

The other gene therapy is cretostimogene and that’s the gene therapy with CG Oncology. That’s not approved yet. We think that they will go in front of the FDA within 12 months, and hopefully, it’ll be approved and used for patients at that time. The results look great, but it’s not approved, so we can’t recommend it at this point.
There’s not one clear drug that’s better for every patient. It’s a personalized decision.
Dr. Ashish Kamat
Current Clinical Trials
Stephanie: We will also talk about clinical trials before the conversation is over and how you explain them to your patients. The term itself is not very friendly, I think. “Clinical trial” is pretty daunting for people, so we’ll humanize it.
There’s already gene therapy approved, like you mentioned, but cretostimogene is still in research. When you’re thinking about gene therapy for patients, let’s say that someone is able to go on to a clinical trial if it was recruiting or let’s say that it was already approved, why is this one better for certain patients than the one that’s already approved?

Dr. Kamat: There’s no one particular drug that’s better for a patient. In fact, when it comes to sequencing the right drug with the right patient, this is a very complex problem. The international bladder cancer group that I lead convened a think tank and spent months thinking about this. We had a hundred faculty members from all over the world go to Houston, sit in one room, and hash this out to try to figure out how to best sequence these drugs for the patients.

The short answer is that there’s not one clear drug that’s better for every patient. It’s a personalized decision. Some patients might benefit from treatment that’s given once every three months and, in which case, it’s obviously the nadofaragene firadenovec-vncg (Adstiladrin).
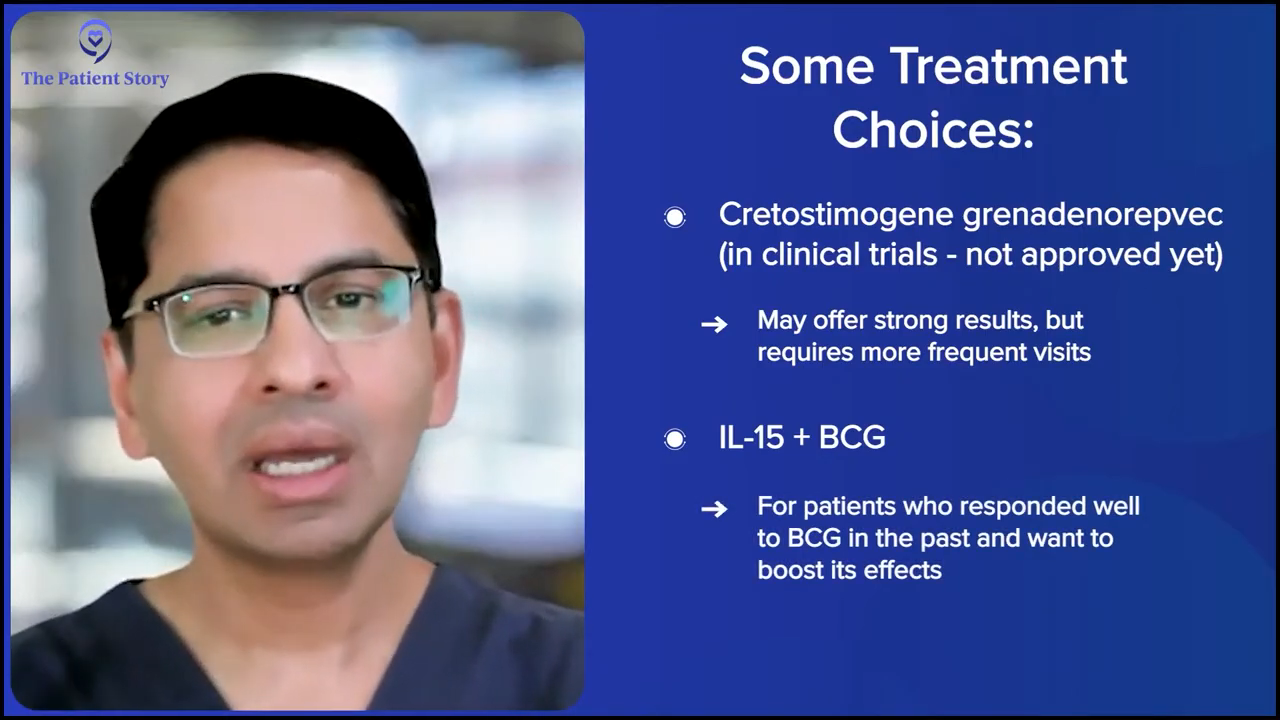
If the CG Oncology’s cretostimogene is approved and it’s available, that’s every week for six weeks and then there’s maintenance that goes with it. But the efficacy numbers there are higher than with the published data with nadofaragene firadenovec-vncg (Adstiladrin), so a patient might say, “I want to try this. I don’t mind coming every so often. I don’t mind the side effects of it. I would like this drug.” That becomes a discussion with the patient.
It’s not just these two gene therapies. Other treatment options are available as well. For example, the IL-15 NAI (nogapendekin alfa inbakicept), patients might like the fact that it’s given in combination with BCG and say, “BCG worked for me. It just stopped working after some time. I would rather get that treatment.”

Then some patients might say, “I don’t want any of this intravesical therapy. I want to get the device that you can put in my bladder because I like the fact that you can put it in, I don’t have to come back for X number of weeks, it’s constantly releasing the chemotherapy in my bladder, and I’m having good results with it.”
I don’t mean to dodge your question, but the real answer is there’s not one treatment that’s the best treatment for everybody.
Stephanie: I appreciate that and I probably should have said it differently. That’s exactly what I think the question is. We’re getting to a space of personalized treatment, not just in terms of the disease, but what a patient prioritizes for his or her life. Again, I appreciate that that’s how you approach your patients and how you bring this to light and at the forefront of these discussions, because it is different. People are optimizing for different things in their lives.
I know you said that they don’t like the pretzel name, but can you talk about the device and whether it’s in clinical trials? We’ll also be putting up the clinical trial names for people in case they are interested in asking their doctors. Do you have the clinical trial name for the gene therapy from CG Oncology?
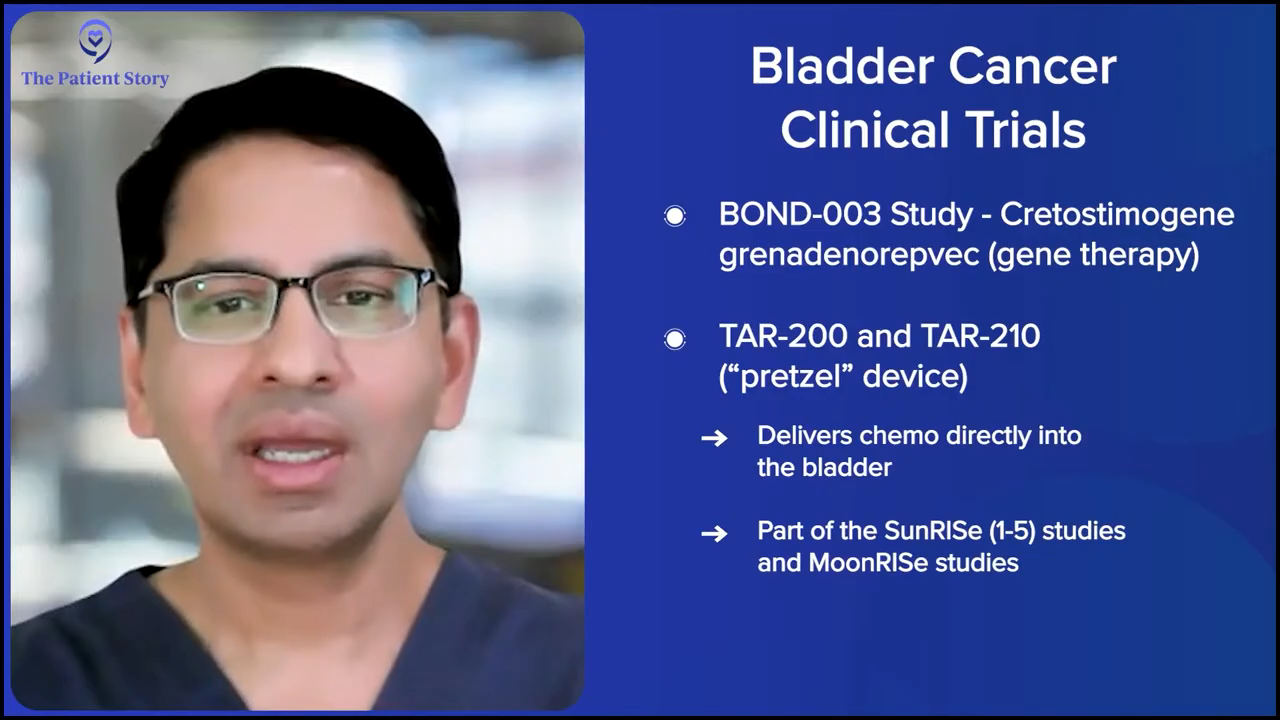
Dr. Kamat: That’s the BOND-003 study. The pretzel is TAR-200 and TAR-210, and that’s Johnson & Johnson’s MoonRISe and SunRISe paradigms, and there are five SunRISe studies. Full disclosure: I’m part of advising pretty much every company in the bladder cancer space, so I help advise all these companies as well. The SunRISe paradigm is SunRISe-1, SunRISe-2, SunRISe-3, SunRISe-4, and SunRISe-5, and then MoonRISe, which is using TAR-210.
We don’t know what the company is going to call this when it actually gets approved in the clinic. There are a lot of names being thrown around and I can’t reveal that anyway, but we don’t know what the final name is going to be. That’s all in the noninvasive space.
I also want to enlighten the patients on the muscle invasive disease and the metastatic disease. In the metastatic space, this is not brand new, but using enfortumab vedotin with pembrolizumab (EV+P) clearly has changed the treatment paradigm. About eight to 10 months ago, we would tell patients with metastatic disease that the median survival is 14 months, which means half the men and women will not live 14 months.
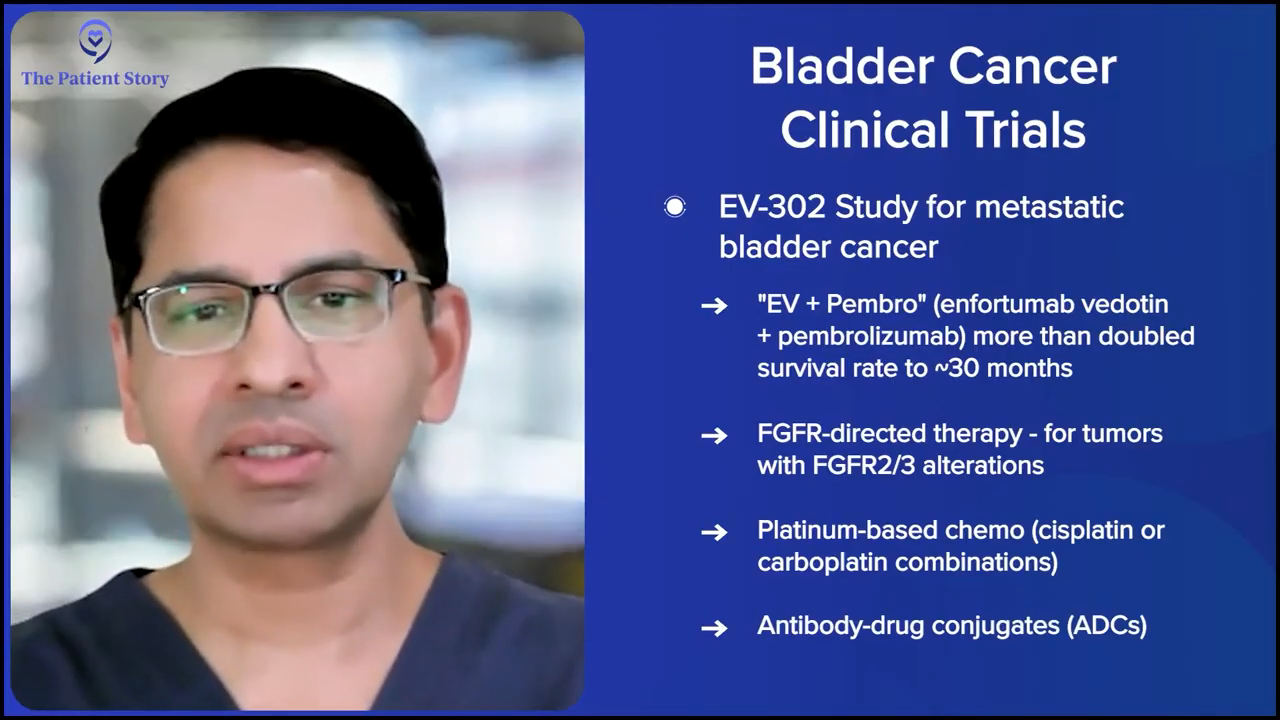
Once EV+P was studied and presented by Dr. Tom Powles at ASCO, he got a standing ovation, as many of you know, because it changed the paradigm for patients. Now we can tell patients who have metastatic disease that the median survival has jumped from 14 months to 30 months, so half the men and women will live 30 months and that’s great.
In the metastatic space, a lot of second-line and third-line therapies are being studied. That’s where you have FGFR-directed therapies, platinum-based combination therapies (cisplatin or carboplatin), and other antibody-drug conjugates (ADCs) that have been developed for patients with biomarkers, etc There’s still a lot of work that needs to be done. It’s not as though EV+P is a full stop. We still need to get patients to live longer than a median survival of 30 months.
Traditionally, what’s happened is if you don’t take the patient’s bladder out, these tumors recur and when they do, they recur with a vengeance
Dr. Ashish Kamat
In the muscle invasive space, where patients have muscle invasive bladder cancer, but they’re trying to save their bladder, a lot of research is being done on whether we can avoid a radical cystectomy. The treatment paradigm there is to give neoadjuvant therapy, whether it’s platinum-based with gemcitabine-cisplatin plus X, and then get the patient to where there is no tumor left in the bladder.
Traditionally, what’s happened is if you don’t take the patient’s bladder out, these tumors recur and when they do, they recur with a vengeance and get worse and then you’ve missed that window of opportunity. Now, with the improved tools that we have where we can use good cystoscopy bladder MRI, using circulating tumor DNA, there is data emerging — and it’s still being studied in a clinical paradigm though — that we might be able to select a subgroup of patients where it might be safe for them to get systemic therapy and not necessarily have the bladder removed. But that, again, to caution people, is a clinical trial paradigm.
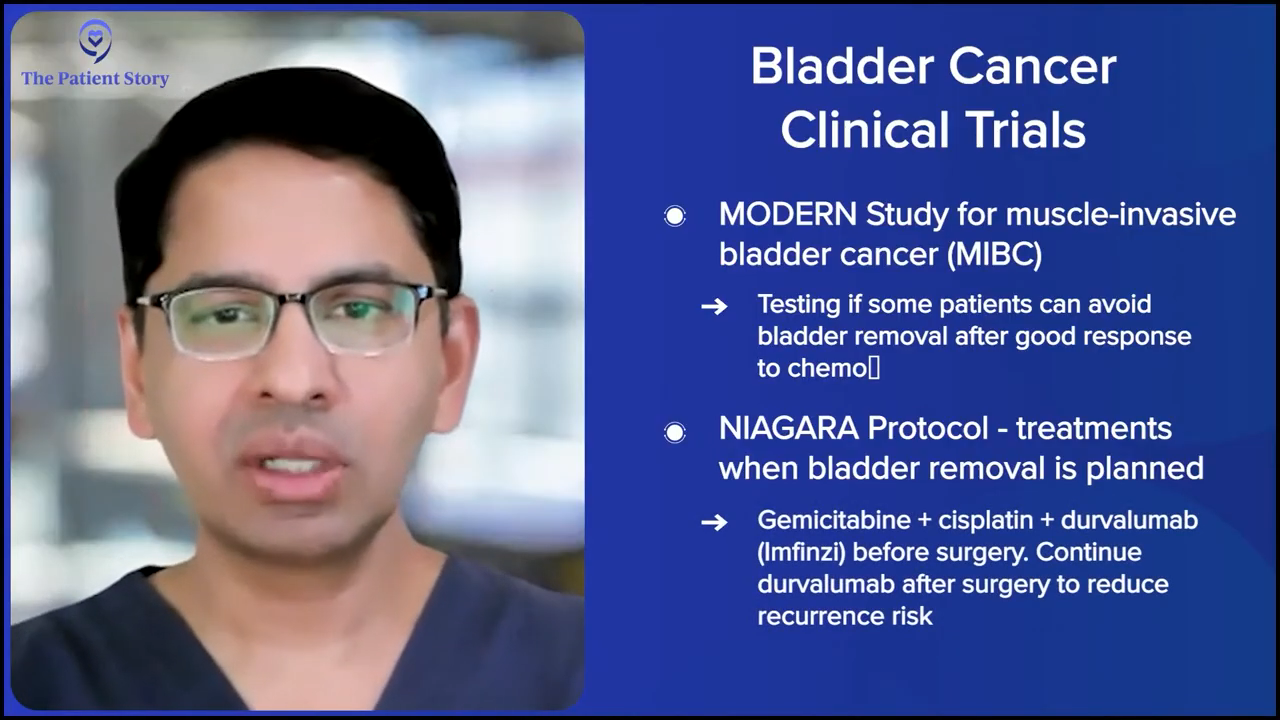
One of the trials being done in that space is the MODERN study. Dr. Matt Galsky is the principal investigator for that. It’s great because that gets patients to see, “I’ve achieved this particular endpoint. Now, on a clinical trial, can I continue on and not have my bladder removed?” That’s the clinical trial part of it.
But in the actual clinic, we are looking to improve results for patients who want to have their bladder taken out. That’s where the NIAGARA protocol, which is using gemcitabine and cisplatin, which used to be used alone as a combination, but now you can combine this with durvalumab (Imfinzi), which is an IO (immuno-oncology). They give this to patients upfront, they undergo radical cystectomy, and you continue the durvalumab (Imfinzi) after the surgery.
This has improved their survival and it’s the first time that the overall survival has shown an improvement in patients who are undergoing radical cystectomy. For many years, we’ve used neoadjuvant therapy and the benefit to patients is about 5%. Nothing has improved upon that. People have tried. They’ve used different types of chemotherapy regimens, dose-dense MVAC (ddMVAC or methotrexate, vinblastine, doxorubicin, and cisplatin), spacing out chemotherapy, etc. This is the first time that this combination therapy has been shown in a randomized phase 3 study to improve overall survival for these patients. Clearly, it changes the treatment paradigm.
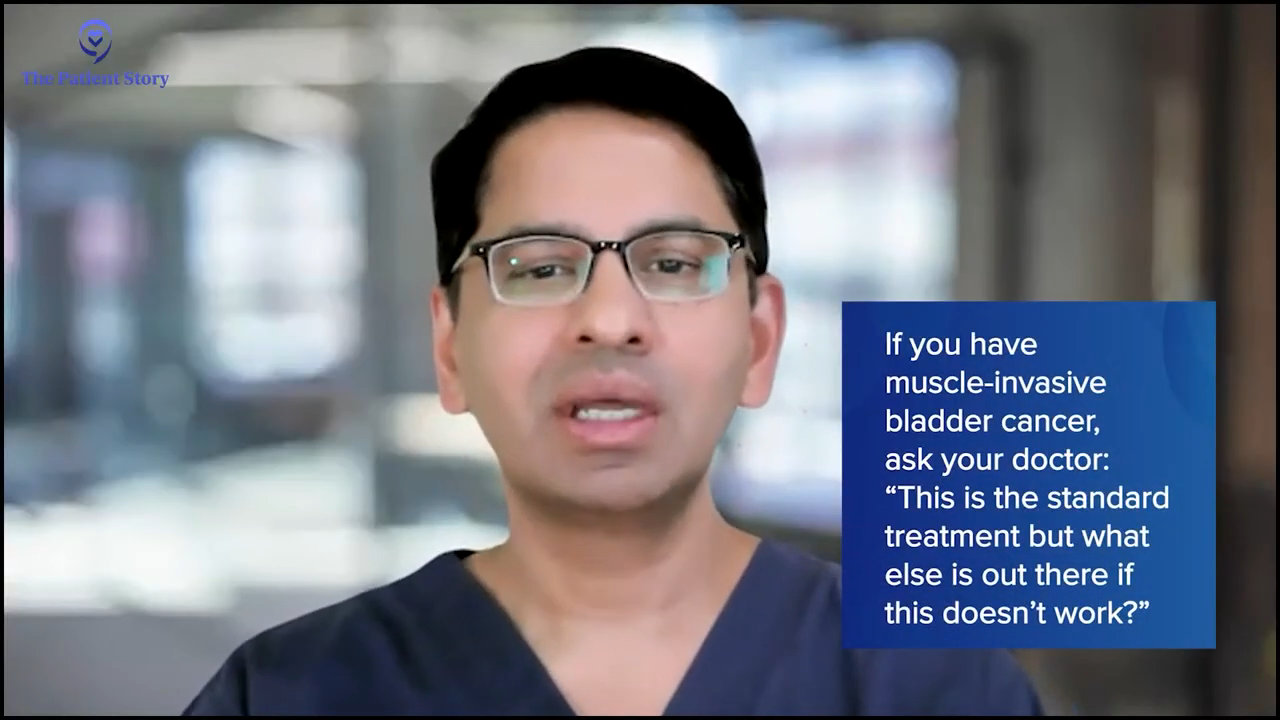
Again, in the interest of time, I’m not going to go into too much detail, but there’s so much in between that is being developed and has been developed. I encourage patients who are faced with the conundrum of having muscle invasive disease to ask their physicians. “I know that this is standard of care for me, but if it doesn’t work, what else do you have available?” Because there’s a lot of stuff that’s available.
Stephanie: Thank you. I’m glad you explained it all in a very concise way, given the time crunch. I do want to go back to some of these because when people are hearing about it, it’s great that they have the information, but there are lots of questions about efficacy and impact on quality of life. You’ve talked a lot about bladder sparing, which is a huge part of what we’ve heard in terms of questions from patients as well, since there is such a high degree of impact on them.
There doesn’t appear to be any major difference between the side effect profiles of one over the other.
Dr. Ashish Kamat
Are New Bladder Cancer Treatments Decreasing Side Effects?
Stephanie: Going back to non-muscle invasive, with gene therapy, you have different considerations. One of them is once every three months and the other is once every week for six weeks. What about side effect profiles? Are you seeing something much better in the one that’s in clinical trial now, for instance, for the quality of life for people?
Dr. Kamat: Again, it’s not as though the side effect profile is majorly different or better or worse in some ways. Patients who will get side effects, you’ll see that upfront. Most patients will have some localized side effects in the bladder, but they’re very manageable and very tolerable, especially since we can give patients antispasmodics, etc. But there doesn’t appear to be any major difference between the side effect profiles of one over the other.
Understanding TAR-200 (“Pretzel” Device)
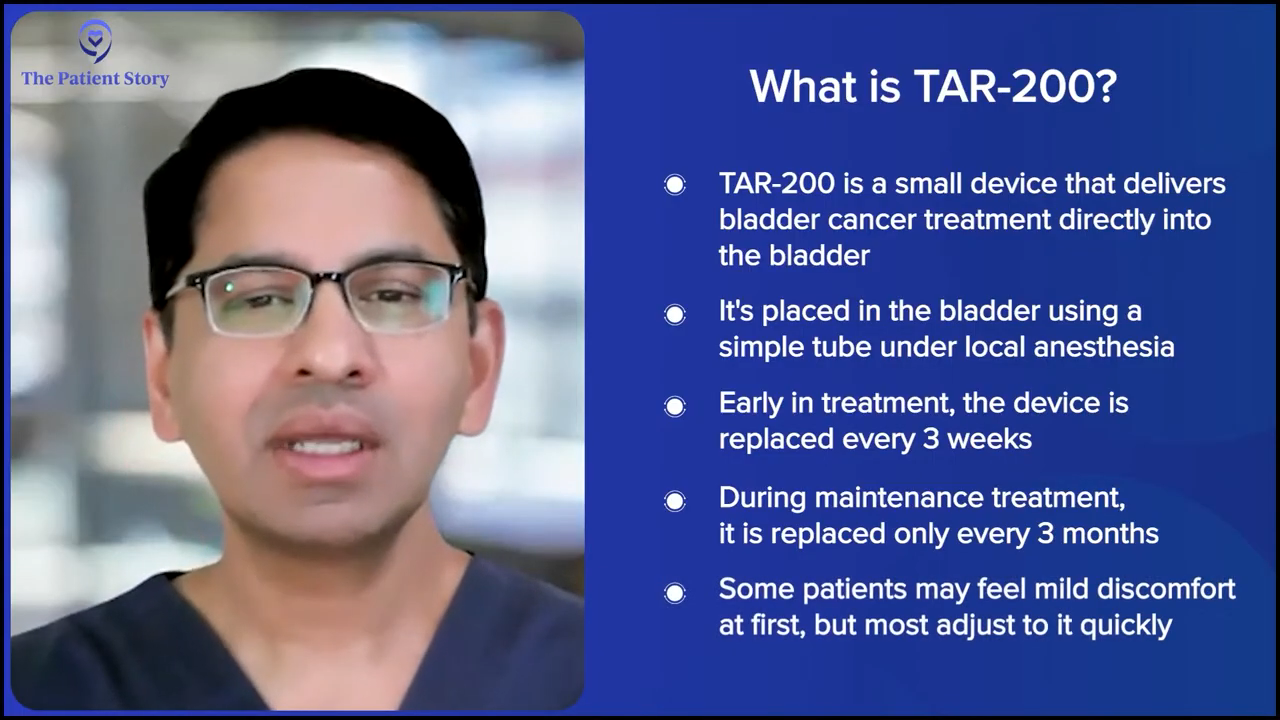
Stephanie: The pretzel device is novel and people will have questions about how this works. Do people go to the doctor or to the hospital and then it’s put in for them and it automatically releases the treatment? How often are you going back in? Is that indefinite or is there a particular amount of time that you’re supposed to have that pretzel device in?
Dr. Kamat: The way the clinical trials are designed is essentially the TAR-200 device, which we colloquially call the pretzel, is put in the bladder with a device that is like a catheter, so it’s not a major surgical procedure. It’s done under local anesthesia. You put it in the bladder and the patient keeps that in place.
Most patients will initially feel like there’s a little bit of a foreign body in there, but patients are, in some ways, used to it because they’ve had prior treatment and it’s something that you can easily treat with an antispasmodic. After a while, patients sometimes forget that they have this in there.
The TAR-200 device, which we colloquially call the pretzel, is put in the bladder with a device that is like a catheter, so it’s not a major surgical procedure. It’s done under local anesthesia.
Dr. Ashish Kamat
Now, in the early stages of treatment, the device has to be changed more often. They come in every three weeks and get the device changed. In the maintenance phase, it has to be changed roughly every three months, so they can leave it in there for that long.
It doesn’t bother most people. Sometimes we have to remind patients that they have a stent in place. We don’t want them to forget that they have it and never come back. Hopefully, that’s a good problem to have, but we don’t want them forgetting that they have it in there.
Like I said earlier, that’s where the discussion on personalized treatment with the patient comes in. Some patients are more than happy to come into the office every so often, while some don’t and prefer to come in once every three months. We’ll have different things to offer to patients, but if we look at the efficacy numbers, it looks like one of these newer agents that are not approved might end up with the best efficacy numbers. We don’t know that for sure yet.
Stephanie: We’re still waiting for that actual data.
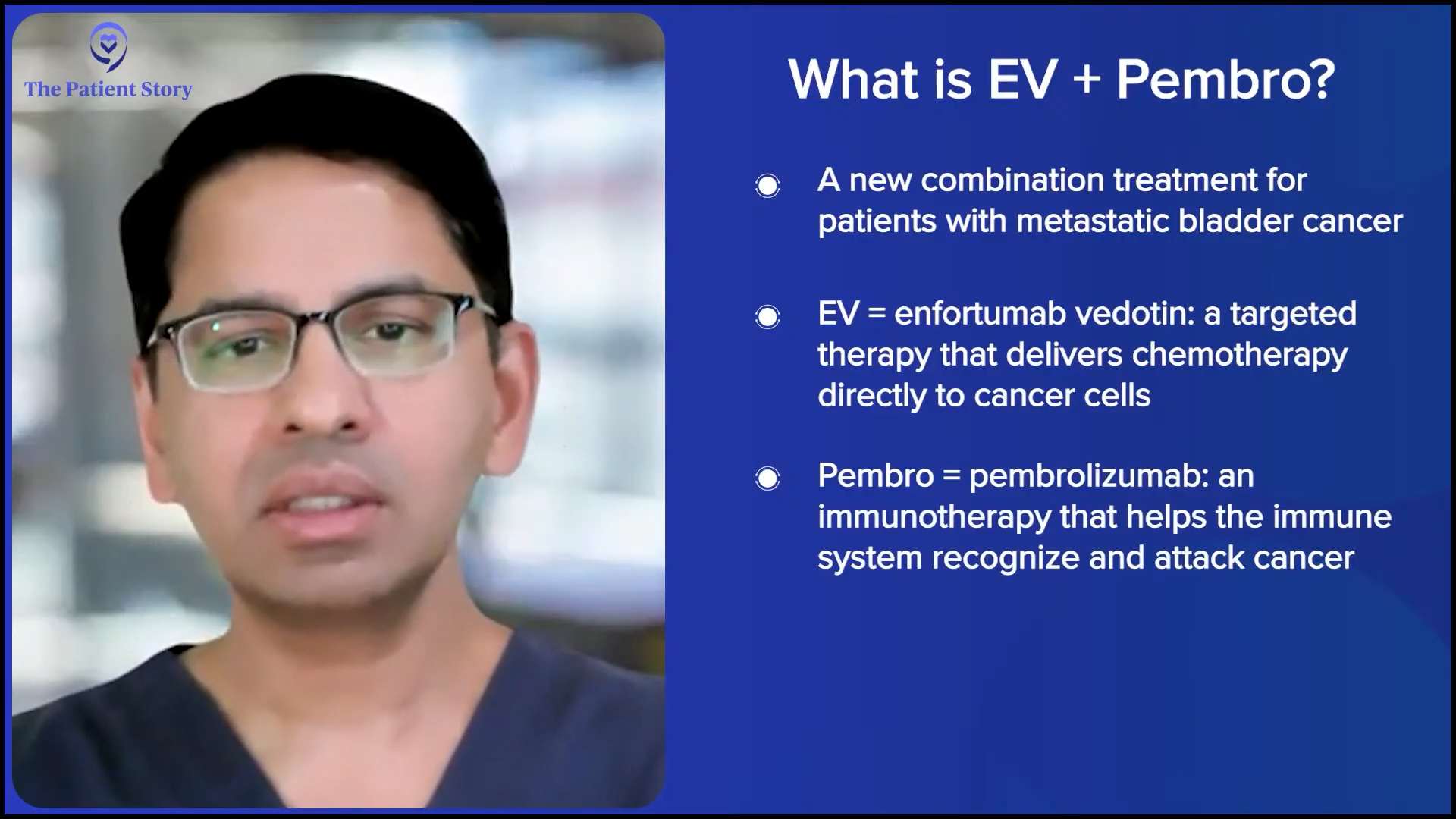
Understanding Treatment with EV + Pembrolizumab (EV+P)
Stephanie: Lastly, as we’re wrapping this, the EV+P, which you’re talking about, the ASCO and the standing ovation, can you put into perspective what this means for patients who typically were looking at pretty rough numbers before?
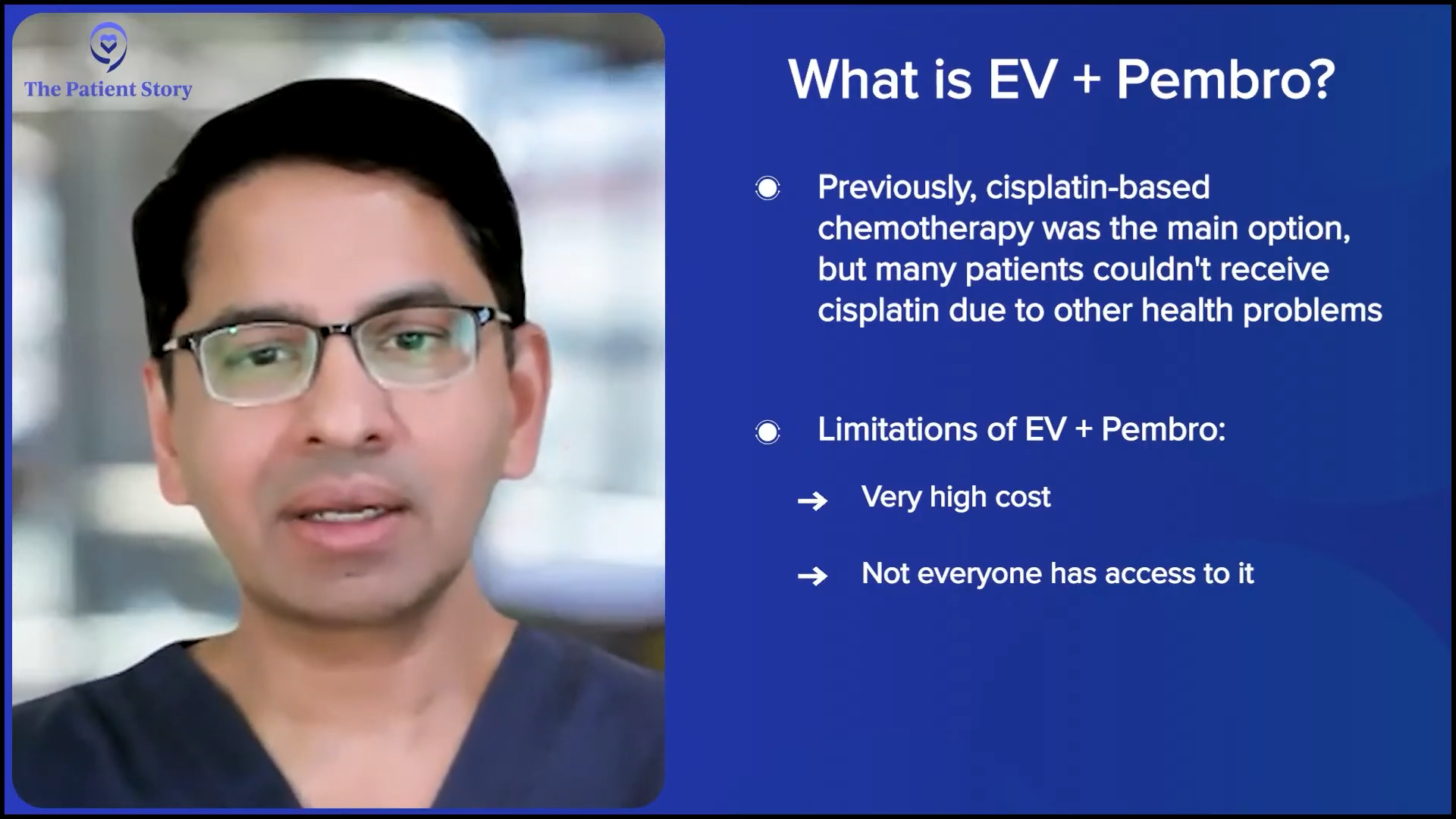
Dr. Kamat: Traditional first-line chemotherapy for patients was cisplatin-based chemotherapy. Many patients could not get cisplatin because of the various comorbidities that our older patients tend to have, especially those with bladder cancer, with prior smoking history, cardiac disease, etc., and they had to get non-cisplatin-based therapy. There was a lot of push to study IO therapies, like pembrolizumab (Keytruda), atezolizumab (Tecentriq), etc. Then the antibody-drug conjugate, which is enfortumab vedotin (Padcev), targets nectin-4.
Long and short, this combination was studied in the patient population and compared head-to-head with standard chemotherapy, and clearly improved survival over standard chemotherapy. Now, it’s become the de facto first-line therapy for patients with metastatic disease.
But a caveat. It’s not available everywhere in the world. It’s expensive. There are many places in the world where patients still don’t have access to EV+P, or if they have access, it is something that can break the bank. Again, that’s a practical problem that we all face: resource allocation. Who gets the treatment? Who can get the treatment? Who can afford to get the treatment? But as long as the patient has access to it and it’s available and affordable, EV+P has become the de facto standard of care.

Humanizing Clinical Trials
Stephanie: We’ve talked about clinical trials. Without going into the details of these specific trials, they’re something that a lot of people are not familiar with. What tends to be the way that you have found most effective in describing what a clinical trial is and why patients whom you bring this as an option to might want to consider it? How do you humanize the concept of a clinical trial to them?
Dr. Kamat: That’s a very important question. Patients need to understand that a clinical trial does not mean that they are being treated like guinea pigs. That’s the furthest from the truth. There are some patients who, unfortunately, don’t have any options because they haven’t responded to anything; at that point, it’s a matter of life or death. It’s either a clinical trial or nothing. But for most patients, that’s not the situation.
For patients in front of me who have different options available, but there’s a clinical trial in the space, what I tell them oftentimes is, “This particular agent that’s being studied in the clinical trial is something that we believe in. We know from early data in the preclinical space that it has good mechanistic reasons to maybe be better than the standard of care at some point. We don’t know that yet. We have to study it.”

The advantage of taking part in the clinical trial for every patient is that they are at the forefront of the research, but they also get better care. If a patient is on a clinical trial, by definition, they are being followed very closely. There’s usually a nurse that’s assigned to them. Everybody’s looking at the pathology very carefully because the company doesn’t want any mistakes. We, as investigators, want to make sure patients are getting the best care across the board, but especially on a clinical trial because there’s that much scrutiny.
Patients need to understand that a clinical trial does not mean that they are being treated like guinea pigs. That’s the furthest from the truth.
Dr. Ashish Kamat
In some ways, the patients are most catered to in a clinical trial. There’s very little room for error. The pathology is often double-checked at a central pathology. Everything’s done on a well-defined protocol and schedule, and it’s made patient-friendly. I often tell patients, “If you take part in the clinical trial, you will be getting the highest standard of care of any patient that’s getting treated for a particular disease. And you’ll not only be helping yourself, but hopefully you’ll be helping inform the field and help other patients as well.”

But we have to be practical. We always tell patients, “Clinical trials are a commitment. It’s not as though you can skip appointments. It’s not as though you can not take part in every part of it. If it’s too much of a social or financial burden, let us know upfront because there’s no obligation.”
I never want patients to feel that if they say no to a clinical trial that I’m recommending, I’m going to take it personally. No, not at all. It’s not a personal thing. It’s me trying to get them to take part in something that I think will help them. But if they can’t participate, they’re not going to hurt my feelings at all and they still will get treated.

Conclusion
Stephanie: Dr. Kamat, thank you for spending time today with us and for everything you do for patients and care partners in this space. We appreciate it.
Dr. Kamat: It was my pleasure. Thank you so much.
Stephanie: I hope this conversation with Dr. Kamat helped you. Please feel free to share this discussion with others in your community.
While we hope that this was helpful and that you walk away with questions to ask your healthcare team, this is not a substitute for medical advice.
Visit ThePatientStory.com if you want an entire library of other patient stories. I’m so glad you could join us and I hope to see you again soon. Take good care.

We would like to thank our promotional parter, The World Bladder Cancer Patient Coalition (WBCPC), which brings together bladder cancer patient organisations from around the globe to improve the lives of people affected by bladder cancer. They are committed to raising awareness, providing trusted patient information, and ensuring the patient voice is heard in research, policy, and care.
Bladder Cancer Patient Stories
Laurent Gemenick, Bladder Cancer
Symptom: Presence of blood in urine
Treatment: Surgery: transurethral resection of bladder tumor or TURBT
Jon T., Locally Advanced Muscle-Invasive Bladder Cancer
Symptom: Darkening urine, blood in urine, dull right flank pain
Treatments: Surgery(transurethral resection of bladder tumor or TURBT), antibody-drug conjugate, chemotherapy
Michael V., Bladder Cancer (Non-Invasive High-Grade Papillary Urothelial Carcinoma), Stage 1
Symptoms: Frequent urination, burning sensation when urinating
Treatments: Surgery (transurethral resection of bladder tumor or TURBT), immunotherapy (Bacillus Calmette-Guérin or BCG treatment)
Dorinda G., Bladder Cancer
Symptom: A significant amount of blood in the urine
Treatments: Surgery (transurethral resection of bladder tumor/TURBT, surgery for papillary lesion), immunotherapy (BCG), chemotherapy
Healing Together: A Mother and Daughter Navigate High-Grade Bladder Cancer
Mary Beth’s story about caregiving starts with an important awareness message about female bladder cancer symptoms.
Danny G., Non-Muscle Invasive Bladder Cancer
Symptoms: Fatigue, back pain, erectile dysfunction, nausea
Treatments: Surgery (transurethral resection of bladder tumor or TURBT), chemotherapy, immunotherapy
Bladder Cancer Resources
Bladder Cancer Causes & Symptoms
Understand common bladder cancer causes, urine color, symptoms, and treatments as described by real patients.
...
Bladder Cancer Series
Bladder cancer patients Ebony & LaSonya talk about their cancer journey, including their first symptoms, how they processed their diagnosis, treatment options, and how they found support. Dr. Samuel Washington, a urologic surgeon, also gives an overview of bladder cancer and its treatments.
...
Diagnosis and Treatment for Bladder Cancer
Learn about the diagnosis and treatment process from bladder cancer survivors and medical experts. Discover diagnosis and treatment options./p>
...