Advanced Endometrial Cancer
Making Informed Treatment Decisions and Accessing Clinical Trials
Endometrial cancer is the most common gynecologic cancer—and for many people, it comes with more questions than answers. In this honest, expert-led conversation, Dr. Brian Slomovitz from Mount Sinai Medical Center in Florida and The Patient Story’s Stephanie Chuang break down the latest in diagnosis, treatment options, and how to have better conversations with your care team.
Learn about early warning signs, key risk factors like obesity and PCOS, and how biomarker testing and clinical trials are changing the standard of care—especially for advanced and recurrent disease.
Topics:
- Warning signs and symptoms to watch for, including post-menopausal bleeding
- Risk factors that raise your chances, like obesity, PCOS, and Lynch syndrome
- How specialists use biomarker testing to personalize care
- Treatment options beyond chemotherapy, including immunotherapy and hormone therapy
- What clinical trials are, who they’re for, and how to access them—even remotely
- Why it’s okay (and important) to get a second or third opinion

This program has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make informed treatment decisions.
The views and opinions expressed in this interview do not necessarily reflect those of The Patient Story.
Table of Contents
Edited by: Katrina Villareal
Introduction
 Stephanie Chuang, The Patient Story: Hi, there! I’m so glad you could join our program, which is focused on endometrial cancer, especially the recurring and advanced stages, and on shared treatment decision-making. How can you play more of a role in making these decisions and also considering whether clinical trials are right for you?
Stephanie Chuang, The Patient Story: Hi, there! I’m so glad you could join our program, which is focused on endometrial cancer, especially the recurring and advanced stages, and on shared treatment decision-making. How can you play more of a role in making these decisions and also considering whether clinical trials are right for you?
My name is Stephanie Chuang. I’m a former cancer patient, a patient advocate, and the founder of The Patient Story. Through my own diagnosis, although it was a blood cancer, I realized that there’s so much medical jargon out there and our goal here is what I wish I had, which is to cut through the noise.
The aim of The Patient Story is to humanize information after a diagnosis through educational conversations and incredible in-depth patient stories and videos. We’ve got hundreds of those with incredible people, so please feel free to take a look at ThePatientStory.com. Our goal is for you to walk away more empowered, whether it’s your own care or your loved one’s care, and in a better position to ask the healthcare team about the best treatment options for you or your loved one.
We want to thank our sponsor, Karyopharm, for supporting our program, which allows us to bring you education from experts at no cost to you. We would like to note that The Patient Story retains full editorial control of our content. While we hope that this is helpful, keep in mind that this is not a substitute for medical advice, so consult with your healthcare team about your decisions.
We always want to hear from you. We know that there’s a lot going in your lives and that it takes a lot to set aside time. We want to make sure that this is worth your while and that it’s truly helping you. Please let us know what we could do better and what you want more of, like what kind of content, from whom, and what topics.

I’m so pleased to be joined by someone who has been a friend of the program. Dr. Brian Slomovitz is the director of gynecologic oncology and co-chair of the Cancer Research Committee at Mount Sinai Medical Center in Miami Beach, Florida. He also holds a professorship in obstetrics and gynecology at Florida International University and is the uterine cancer clinical trial lead for GOG Partners. His clinical expertise spans so many spaces. We’re talking immunotherapy and clinical trial protocols, where he’s led critical studies in ovarian and endometrial cancer. Dr. Slomovitz is also involved with so many patient advocacy groups and efforts.
Thank you for making the time to join us. We know how busy you are and you have so many of your own patients. Dr. Slomovitz, what drives you to do things like this where you’re helping not just your own patients in the clinic, but the many out there who are looking for more information?

 Dr. Brian Slomovitz: Thank you very much for having me. I appreciate the opportunity, Stephanie, to chat with you and The Patient Story audience.
Dr. Brian Slomovitz: Thank you very much for having me. I appreciate the opportunity, Stephanie, to chat with you and The Patient Story audience.
When we talk about what we do as gynecologic oncologists, our responsibilities don’t end with clinical care. They also include research — and we’ll be able to talk about that more — and educating our trainees, fellows, residents, and medical students. I always say the best way to cure cancer is to prevent cancer. Endometrial cancer, specifically, has some risk factors. It’s important to educate the community of non-diagnosed patients called previvorship and diagnosed patients to make sure that they ask the right questions from their doctors to make sure that they get the best care moving forward. Ultimately, that’s what’s going to help save lives. I’m passionate about this. We went into medicine to do what’s best for our patients. Fortunately, I’ve been given the opportunity to work in this setting to try our best to eradicate this disease.
Stephanie: I appreciate that and I know our patient community does as well.

Signs and Symptoms of Endometrial Cancer
Stephanie: Since you mentioned it, can we spend a brief moment talking about the signs and symptoms of endometrial cancer? We’ve talked to so many people who’ve had, unfortunately, a long path to getting the diagnosis. It may be helpful to understand some things to watch out for and some of the questions people can ask their doctors when they start to feel unwell.
Dr. Slomovitz: That’s a great question. Fortunately, most women with endometrial cancer are diagnosed with early-stage disease. Obviously, there’s a large subgroup that have advanced or recurrent disease that we’re working on with better systemic therapies and clinical trial options. But the reason why we have mostly diagnosed with stage 1 disease is postmenopausal bleeding or abnormal uterine bleeding.
If there are any signs of abnormal bleeding, we educate our patients to see their healthcare providers. Oftentimes, it could be nothing. It could be a polyp. In a premenopausal woman, it could be an abnormal period. But there are also cancers and pre-cancers that need to be diagnosed and that could be done by a simple office procedure. It’s important.

Questions to Discuss with Your Doctor
Stephanie: For those who are newer to understanding this diagnosis, let’s talk about the different kinds of people you’re seeing. By the time they see you, they’ve been referred to a specialist. What are the first questions you ask people you see to help understand how to make the best treatment recommendations?
Dr. Slomovitz: Endometrial cancer is becoming a more and more heterogeneous disease and we’re getting better at dividing these patients into subgroups. When a patient comes into my office with a diagnosis of endometrial cancer, the first thing I think of is, “Why did that patient get endometrial cancer?” There’s a large subgroup of patients that have the standard endometrial cancer or endometrioid endometrial cancer, which is driven by obesity. Fat cells in the body produce estrogen, so the heavier a person is, the more likely they have higher estrogen levels. The estrogen feeds the lining of the uterus or the endometrium, which could transform it into a cancer or pre-cancer.

When we talk about the opportunities for prevention, weight loss is a tremendous opportunity. And in 2025, we have GLP-1 receptor antagonists, like semaglutide (Ozempic) and tirzepatide (Mounjaro), which I’m a big advocate of, especially for women who tried other techniques to lose weight. If they don’t work by decreasing one’s weight, you could decrease the risk factors for endometrial cancer. We’re still doing definitive studies, but I can’t imagine that they’re going to be negative.
Other risk factors include family history. There’s a hereditary predisposition to endometrial cancers in something called Lynch syndrome. It’s a hereditary syndrome that could lead to either endometrial cancers or even colon cancers, and a small group can get ovarian cancer as well.
In strong family histories, we’re looking for history of polycystic ovary syndrome (PCOS), which can lead to endometrial cancer. And there’s always the sporadic instance — or, should I just say, bad luck — that can lead to it. In the office, we want to try to figure out why they showed up, what brought them there, and what risk factors were involved.

How Important is It to See a Specialist?
Stephanie: We’re going to be talking about the different research and the latest in terms of treatment options, especially for advanced recurrent endometrial cancer patients where we know that there were limited options in the past. How important is it to see someone like you who also does so much research outside of seeing patients in the clinic?
Dr. Slomovitz: We want to make sure our patients see a subspecialist in gynecologic oncology. To get to where we are today, I did four years of residency in OB/GYN and we do three or four years of fellowship. I did a four-year fellowship at MD Anderson. That’s all after med school. We’re passionate about what we do.
Seeing a clinician who does a lot of this is important. But I’m passionate about research. We’re not going to rest until we get 100% cure rates. It’s important to talk to your doctor about clinical trial options and what clinical research is going on. It’s important to always get the right opinion, which means it’s okay to get a second opinion or sometimes even a third. It doesn’t always have to be your own doctor who does it, but at least in partnership or one who collaborates with a researcher in the community to make sure that we give the best treatment options to our patients. The best outcomes come in those patients who had the opportunity to do research.

Partnerships Between Specialists and Community Providers
Stephanie: I love that you mentioned working with community providers because I don’t think we hear a lot about that. But it’s about having a full team, like having someone like you who does a lot of research partnered with a community oncologist. A lot of people might not live close to where you practice. Is there a way that they can still access being under your care? It sounds like that happens.
Dr. Slomovitz: I’m in Miami. I’m fortunate to get several patients from South America, Central America, and even Europe. A lot of these trials are only available in the United States, so patients with resources oftentimes come to seek out some of these trials.

The Role of Biomarkers in Endometrial Cancer
Stephanie: Before we get to clinical trials, Dr. Slomovitz, we’ve been hearing a lot about more personalized medicine and understanding how that can help you better make suggestions on treatment. We talk about biomarkers. What are the key biomarkers? Could you describe what that is in layman’s terms and why they matter, especially in advanced and recurrent endometrial cancer?

Dr. Slomovitz: Let’s take a moment to talk about this. Back in 2011 to 2012, there was something called the The Cancer Genome Atlas (TCGA), which was created to do full genetic sequencing of tumors. Now, out of all of the solid cancers that were investigated, endometrial cancer was the one that was able to delineate the disease into four distinct subgroups, something called microsatellite instability (MSI) or deficient in MMR (MMRd) proteins and something called POLE mutations. Those two groups respond well to immunotherapy. The other two groups are called no specific molecular profile (NSMP) or p53 abnormal. Those are the ones that don’t respond to some treatments and need other things. In general, those are the four subclassifications that we look for in endometrial cancer.

In addition to mismatch repair proteins, we’re also looking for estrogen receptor status, progesterone receptor status, a protein on the cell called HER2 is also important when we come up with treatment options, and p53 mutation status. These are biomarkers that we use. They’re signatures that can change the diagnosis from endometrial cancer to a p53-mutated, estrogen receptor-negative, and a microsatellite stable tumor. With those classifications come better treatment options because we define our treatments by the different molecular subclassifications, which is totally different than what we used to do. Not all treatments are the same for different patients.
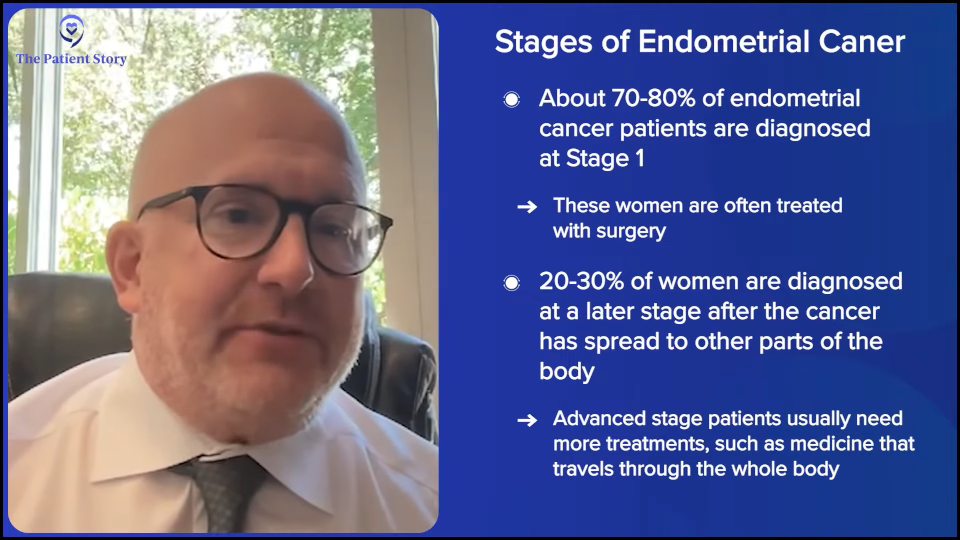
Stephanie: A hundred percent. And you mentioned, thankfully, that a lot of the initial diagnosis in endometrial is stage 1. We also know, though, that there’s recurrent. Can you break down approximately how many people develop recurrent disease and how many are diagnosed at an advanced stage from the beginning?
Dr. Slomovitz: To lean into that answer, there are more women now who die from endometrial cancer than from ovarian cancer. We used to think ovarian cancer is the silent killer. We’re not sure exactly why there’s an increase in incidence. We’re doing a better job in identifying some endometrial cancers, particularly across diverse populations, but it’s a deadly disease.

The standard of care is a hysterectomy. After hysterectomy, we find about 70 to 80% are diagnosed with stage 1 disease. It’s the other patients who were more worried about — the patients with lymph node involvement or whose disease has spread to the cervix or other parts of the abdomen. Those are the advanced-stage patients and require systemic therapies.
Of those patients, the recurrence rates could be quite high. With patients who have advanced recurrent disease, recurrence rates could be 60 to 70%. Again, leading it to the fact that it’s the deadliest of all gynecologic cancers, even though 70% could be treated with surgery alone, it’s the other 30% that we’re worried about. They could be advanced stage and also high risk. Some of the high-risk histologies, such as the p53-abnormal group, are the ones who are set up for recurrent disease as well. We need to come up with more systemic treatment options.

Understanding Your Treatment Options
Stephanie: Is there anything else that you want patients to know by the time they see you and they have either advanced or recurrent endometrial cancer? What are the top things you want them to understand about the landscape of treatment options and what’s available?
Dr. Slomovitz: It’s important to make sure that we offer the best standard of care for our patients, and to take the time to explain to our patients what the best treatments are and what clinical trial options there are available. It’s important.
A lot of times, patients and their families say, “Let’s get started on treatment and then find options.” I would rather wait a couple of weeks to start on a treatment option to make sure it’s the correct treatment option. Oftentimes, when you start, you can’t go back to say, “Oh, forget it. We shouldn’t get the chemo. We need to enroll you in this clinical trial.” Where you start your therapy and the options at that point are crucially important because it may eliminate your eligibility for a clinical trial.

Standard of Care for Advanced and Recurrent Endometrial Cancer Patients
Stephanie: What has been the gold standard in terms of standard of care for advanced and recurrent endometrial cancer patients?
Dr. Slomovitz: This has recently changed. Initially, it was just chemotherapy, sometimes with or without radiotherapy. Now in March 2023, two presentations were presented at the Society of Gynecologic Oncology meeting. The studies were very similar where they added immunotherapy to chemotherapy in the first line management of this disease.

Both studies were positive. There were actually two other studies that were also positive and with similar designs. It was chemotherapy plus/minus pembrolizumab (Keytruda), plus/minus dostarlimab (Jemperli). There were other drugs that were investigated: atezolizumab (Tecentriq) and durvalumab (Imfinzi).
All of those studies show that immunotherapy works in women’s endometrial cancer. From that day forward, it was at least a standard of care option for our patients, particularly in that subgroup of patients with microsatellite instability (MSI) or defect in a mismatch repair gene, or in layman’s terms, a certain molecular profile.

If patients didn’t get immunotherapy in the first line, studies have shown that immunotherapy also works well in the second line. When we talk about first line management, that’s the standard of care, but there are also other clinical trials that we’re looking at looking at where treatments may be better. There’s a subset of patients that may be eligible for less than chemotherapy — hormonal therapy — for this disease. That’s a smaller subgroup. But in general, I would say the best treatment options are chemotherapy with immunotherapy.
Stephanie: To follow up on that, who makes up that small population who benefits from hormonal therapy?
Dr. Slomovitz: It’s a subgroup of patients that have less aggressive disease. Maybe they have metastatic disease, but it’s just in one area that maybe has an indolent or slow-growing growth pattern and is estrogen receptor-positive. It doesn’t mean we’ll eliminate chemotherapy, but we may start those patients off with non-chemotherapeutic options.

In some patient populations, we’re also studying immunotherapy in the first line without chemotherapy. Some of those studies haven’t been read out yet, but there’s one study that I’m leading globally with pembrolizumab (Keytruda), in the dMMR patient population with advanced recurrent disease, giving immunotherapy alone versus chemotherapy alone to see if that’s actually better.
Stephanie: It’s exciting that there are so many things happening in this space. Historically, what have been the issues with all the chemotherapy? I know a lot of this is about quality of life. What would you have to talk to patients about in terms of helping with that?

Dr. Slomovitz: One of my career goals is to get rid of chemotherapy. Why? The response rates of chemotherapy, even in the first line, are about 50 to 52%. The duration of response or the progression-free survival is only about 14 months. Those numbers aren’t good enough.
There are side effects of chemotherapy. Improving efficacy while maintaining or improving quality of life is our ultimate goal, and we can see that hopefully with immunotherapy. If the studies are positive about getting rid of chemotherapy, that’ll be super exciting. And if the studies on hormonal therapy and biomarker-driven therapies that are ongoing are positive as well, that would also be exciting to hopefully get rid of chemotherapy.

How to Have Better Conversations About Treatment
Stephanie: Is there anything else in terms of standard of care right now? You’re at a large center and you’re a specialist. Are you hearing from patients though who’ve been in the community who are still getting only chemotherapy? What are the things that we can be doing as a patient group to help make sure that the right treatment option conversations are happening?
Dr. Slomovitz: That’s where some of the frustration stems from. At my center right now, I have one study in the adjuvant setting before it even spreads. I have two studies in the first line setting to give the best treatment in the first line. And I have several studies, maybe seven or eight, in the second or third line setting for this disease.
It’s okay to get good standard of care. I don’t think clinical trials are for all patients, but there are some patients who are super motivated and want to improve their treatment options. What we’re using in the first line is some of the second-line therapies that have been proven to work and moving them to the first line to see if they work better in the first line.
We’ve seen that a lot of times. Chemotherapy works better the first time you give it. Hormonal therapy works better the first time you give it. With some of the novel therapeutics, by pushing them to the first line, it may be more beneficial to our patients. But we don’t always get referred those patients. It would be nice if we did.

Stephanie: Part of this is trying to get the attention of people to let them know that these options exist and that there are particular places you’ll need to go most likely to get access to them and to these clinical trials.
Dr. Slomovitz: Stephanie, that goes back to your first question. Why do I do what I do? Why do we do this? Because we have to get the word out. We’re hoping that your audience may have a family member who says, “Oh, wait a second. I live in New York, so I need to go to a major medical center in New York. I live in Florida. I need to go to the center here.”
The group that I work with, the Gynecologic Oncology Group, is a national organization. We have international partners as well of over 350 sites. Our goal is not to get the best clinical trials, but to get the best clinical trials nationwide so patients don’t have to travel so far for their care. They don’t need to go to some of the best cancer centers in the world. They can get it locally, closer to home.
Stephanie: I love that. I love that there’s an effort to get access out there to more people.

Asking Your Doctor About Biomarkers
Stephanie: You’ve talked about biomarkers. You’ve talked about different groups, how certain groups do better, and how studies show how immunotherapy performs. Other groups need to have other options. What questions should people ask, especially those who are unfamiliar with their diagnosis? What can they ask their local doctor about testing?
Dr. Slomovitz: Biomarkers in general could be prognostic, meaning they could tell how well a person’s going to do or predict a certain response. We also have specific biomarker therapies as well.
In talking to their doctor, they need to be somewhat educated. “Doctor, what’s the best therapy out there? What are the best therapies that are being investigated? Are there any treatments for my biomarker signature tumor? Are there others that work in general?” Having that lingo down makes a lot of sense. If they go to their doctor and the doctor says, “We don’t have clinical trials in this area,” my work would be considered not successful then.

We have clinical trials available. We have a lot of clinical trial options available for our patients that we want to talk about. They should talk to their providers about it and make sure, to the best of our ability, that the patients at least see a provider who offers clinical trials, even by telemedicine if they’re calling from a distance. In Florida, we can do telemedicine visits that could help educate patients on options and also educate us to see whether or not a patient’s eligible for these trials.
Stephanie: You mentioned you work with patients from everywhere, so you’re saying telemedicine is still available and you’re able to consult with or provide some medical guidance to people in other states.

Dr. Slomovitz: There are certain billing rules there as far as the ones we can formally see within the state of Florida, but even for patients who are calling internationally, we try to give an opinion to see whether or not there are options for the patients. For example, sometimes the trial may not be open here, but it’ll be open in another area of the country and that they’re able to get those options.
For a cervical cancer trial that I had, patients came from Argentina. Not only is the drug not available in Argentina, the trial wasn’t available. In the United States, oftentimes we have access to some agents and trials that aren’t available globally.
Stephanie: I’m so glad to hear about that access.

Humanizing Clinical Trials
Stephanie: Let’s dive right in. How do you describe clinical trials as an option to your patients, especially those at an advanced stage? They’re probably feeling pretty overwhelmed. For those who have recurrent disease, they’ve already gone through treatment and now they have to go through something again. How are you having that conversation with them and describing it?
Dr. Slomovitz: The first thing is we discuss, as far as what they could possibly get, is their medical history, what they’ve already gotten, and where they stand. Over the last couple of years, we’ve learned that immunotherapy is a good option for our patients. A lot of the trials that we’re writing now require prior immunotherapy because we don’t want to test something until they’ve gotten the best care so far.
Then as we move forward into what trials are available, clinical trials are almost like a cookbook. They need to follow the recipe. They’re looking for drug registration. They’re looking to be compliant with the FDA guidelines. There are specific screening or eligibility and exclusion criteria that we go through with our patients to see if, in fact, they’re eligible.

Some of those criteria could be how many lines of therapy, what biomarkers are present on the tumor, and other medical comorbidities or problems that may limit them from being in the trial. It’s all about getting to know our patients and seeing what options there are. There’s a new drug class called antibody-drug conjugates (ADCs). They’re chemotherapies but they’re targeting specific proteins on the cell.
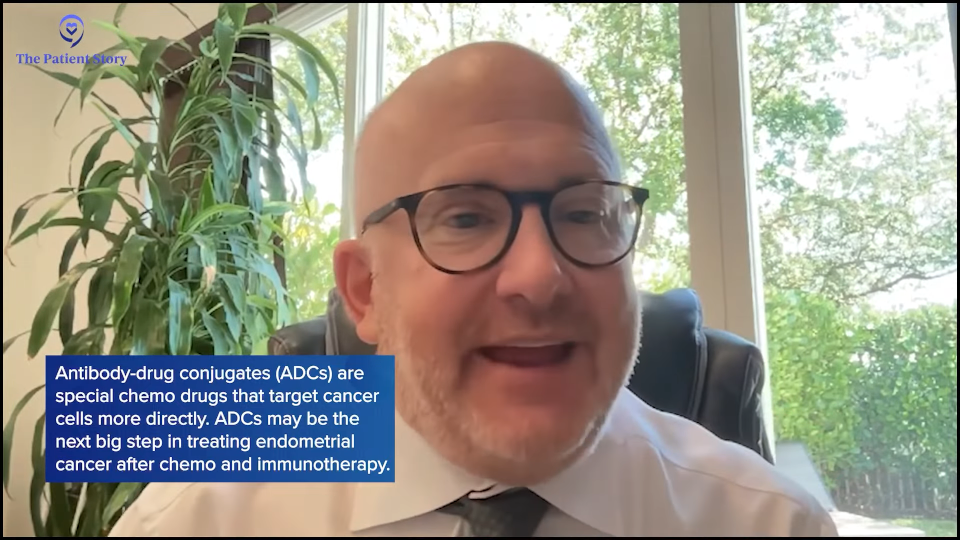
I believe that’s going to be the next line. We talked about immunotherapy and chemotherapy being used. The next is going to be what the best agents are to use after chemo and immunotherapy, and I do believe a large component at least will be these antibody-drug conjugates. They’re under investigation now. We have approximately nine to ten trials looking at antibody-drug conjugates. They have different targets and sometimes different chemotherapy attached to them, but we’re looking to see what the best ones are for our patients.
As we move past that, we need to see. Right now, we’re doing ADCs post-immunotherapy and post-chemo. At some point, it’ll be a day when patients receive an ADC as well, so we’re already doing those studies to see what the best treatments for those patients are.

Highlighting Key Clinical Trials
Stephanie: It sounds like there’s so much movement. If we were to break this down so we can talk about specific clinical trials, the ones that you find the most promising or exciting, and break it down into the subgroups that you talked about, how would you highlight the top clinical trial for microsatellite instability-high (MSI-H) patients?
Dr. Slomovitz: The tumors that we talked about are very amenable to immunotherapy. I alluded to this, but what can we do to get rid of chemotherapy? We’re giving those patients immunotherapy alone. This is a trial called KEYNOTE-C93. Hopefully, it’ll read out in the near future. But in patients with microsatellite instability, we’re giving them chemotherapy versus immunotherapy alone. If that’s a positive trial, it’ll be a game changer.
Now we’ve seen that already in microsatellite instability colorectal cancers. A couple of years back, there was a study at Memorial Sloan Kettering where 13 patients with microsatellite instability were treated with dostarlimab (Jemperli) and all of them responded. More recently in 2025, presented at the ACR meeting was a study of patients with microsatellite instability. There were colon cancer patients and other diseases as well, including endometrial. Not only did the immunotherapy work, but we were able to avoid surgery in a subgroup of patients.

Now forget about getting rid of chemotherapy. Imagine if we get rid of surgery as well. How great would be that for a patient?
One of the areas I think we need to do better now is the microsatellite instability subgroup. We know that immunotherapy works, but it doesn’t work for some subgroups of patients. What’s the role of giving immunotherapy after immunotherapy? And that’s an issue that we’re investigating.
Stephanie: Can you talk about that a little more? You’re saying for the subgroup where immunotherapy doesn’t work, a second line of a different kind of immunotherapy might work?

Dr. Slomovitz: We’re wondering whether we can give a different immunotherapy or at the time that it fails, adding another drug to the immunotherapy. For example, this drug called lenvatinib (Lenvima). We know lenvatinib (Lenvima) works well with pembrolizumab (Keytruda). The immunotherapy in lenvatinib (Lenvima) is a targeted therapy in patients with proficient MMR or microsatellite instable. One of the things that we’re finding in patients with microsatellite instability that respond to immunotherapy alone is when immunotherapy starts to fail, we adding lenvatinib (Lenvima) and we’re seeing some responders. That’s another option for our patients.
Stephanie: Is there a clinical trial name for that one?
Dr. Slomovitz: Right now, it’s based on an anecdotal evidence case series. But we’re going to be looking at it more closely.
Stephanie: Got it okay. And again, that’s KEYNOTE-C93?

Dr. Slomovitz: That’s the study that I’m doing with pembrolizumab (Keytruda) versus chemotherapy. There’s a similar trial being run in Europe called DOMENICA, which is dostarlimab (Jemperli) versus chemotherapy for microsatellite instable or dMMR patients.
Stephanie: That’s great. Is there anything else in that group that you want to highlight? If not, we could move on to a different subgroup that you want to highlight some clinical trials for.
Dr. Slomovitz: That’s the key message in that population of patients. Let’s move on to pMMR or proficient mismatch repair. There’s an adjuvant trial that’s about to open, but it’s in the public domain, in patients with HER2-expressing tumors at an early stage, so they just had surgery. We know the standard of care there is chemotherapy. We’re going to try chemotherapy compared to an antibody-drug conjugate that targets HER2 protein. If that’s positive, we can maybe push antibody-drug conjugates into the adjuvant setting, not even the first line.

Now in the first-line setting, we have two trials that are very exciting. Both of them are incorporating antibody-drug conjugates. One of them is in the treatment arm. It’s a three-arm study. The DESTINY-Endometrial01 trial is sponsored by AstraZeneca and led by Vicky Makker, a professor at Sloan-Kettering. We’re investigating the combination of chemotherapy with immunotherapy.

Remember that afternoon in March 2023 that I said changed the world? That’s the standard of care. The other two arms were combining immunotherapy plus an antibody-drug conjugate. For HER2-expressing cells, they are also seeing if either of those two arms works. Super exciting. If they’re positive, we get rid of systemic chemotherapy in those patients.

Another similar trial that we’re doing is DESTINY-Endometrial02, which is looking at antibody-drug conjugates not in the treatment setting, but more in the maintenance setting. After patients respond to chemotherapy and immunotherapy, one of these antibody-drug conjugates are given alone or with immunotherapy to help keep it away.

Another exciting first line trial, XPORT-EC-042, which is ongoing, is another maintenance trial. It’s looking at patients who respond to chemotherapy without immunotherapy, giving a drug called selinexor (Xpovio), which is a nuclear transport inhibitor, to try to keep the disease away longer.Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

Right there, I described an adjuvant study and three first-line studies for the management of this disease. How cool is that? Forget about the dMMR setting, which we talked about two other studies that are ongoing. There are a lot of different treatment options for our patients just in the clinical trial world.
Stephanie: That’s incredible. Can we do a deeper dive on what you’ve just described? Because I think there’s a lot to uncover there. These are all in the pMMR group, is that right?
Dr. Slomovitz: Most of the trials that I described are pMMR. Some of them may allow dMMR, but are not going to accrue. dMMR patients are getting immunotherapy. It works. There’s a 70% reduction in recurrence or deaths due to the disease. It’s the pMMR subgroup, the proficient mismatch repair, where we need to come up with better treatment options.
Stephanie: What percentage of the endometrial cancer patient population is in the pMMR group?
Dr. Slomovitz: About two-thirds or 70% are pMMR, which is a good percentage.

Understanding the Role of the HER2 Mutation
Stephanie: Let’s break it down again. HER2 is often linked to breast cancer. What would you like to say about patients who have HER2-expressing tumors? Is there a particular patient profile? Are there other questions that they can ask? I know we talked about biomarkers, but we didn’t go into the testing required and when that can happen.
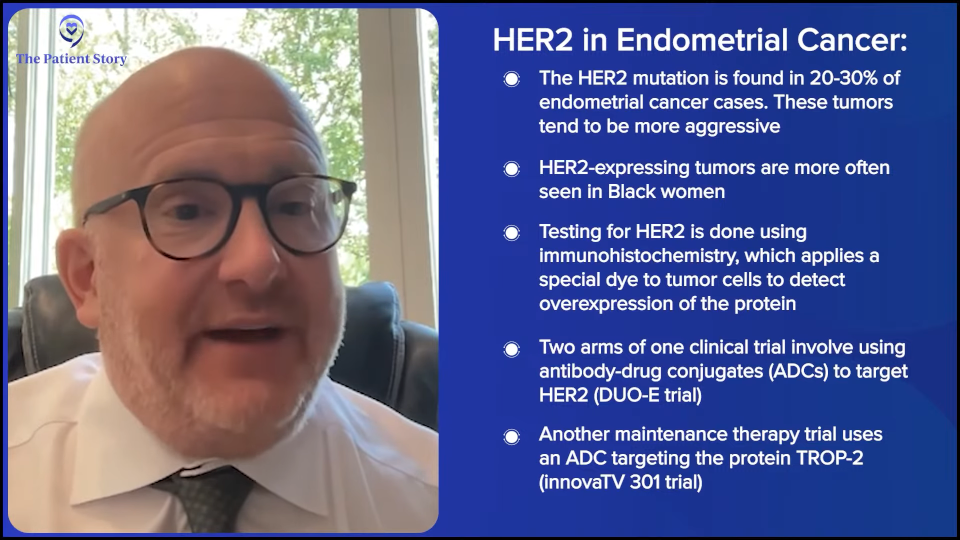
Dr. Slomovitz: I’m glad you brought that up. In one study, DUO-E, two of the arms involve antibody-drug conjugates. HER2 happens in 20 or 30% of the patients and usually portends a worse prognosis. These are the ones with a higher, more aggressive histology.
Interestingly, we’re finding that this more likely affects women of diverse backgrounds. Black women are twice as to die from endometrial cancer than white women. And these HER2-expressive tumors, we oftentimes see in Black patients as well.
Now, the other study, the maintenance trial that I discussed with the antibody-drug conjugate, is with a drug called sacituzumab tirumotecan (sac-TMT). It allows for all comers and it’s not biomarker-driven. It’s an antibody-drug conjugate against TROP2 (trophoblast antigen 2) that’s found on most cancers. But the study doesn’t have to pre-qualify itself by having overexpression. When we talk about what makes it positive, it’s immunohistochemistry. It’s basically putting a dye on the tumor cells that’s attracted to a protein that would be on the surface and the dye needs to be overexpressed either mildly to heavily.
Stephanie: Is the one that’s being led out of Memorial Sloan Kettering? Is that the one you’re talking about?

Dr. Slomovitz: The HER2 study sponsored by AstraZeneca is DE-01. That’s the study that’s being led out of Dr. Vicky Makker from Memorial Sloan Kettering and it’s being run through the GOG in partnership with our colleagues in Europe called ENGOT or the European Network for Gynaecological Oncological Trial. The TROP2 study is a Merck-sponsored trial being led by Dr. Bradley Monk, who’s an investigator at Florida Cancer Specialists. The selinexor (Xpovio) trial, the nuclear transport inhibitor, is being led by Dr. Rob Coleman out of US Oncology. The adjuvant trial is by Dr. Cara Mathews at Brown University.
Part of my job is to make sure we get a lot of different investigators involved with these trials. I’m proud of the fact that we’re training our next generation of gynecologic oncologists on what we need to do for our patients because we want to make sure that this research continues.

The Science Behind Selinexor
Stephanie: Let’s give a layman’s view of the science behind some of this. You talked about immunotherapy really well. You talked about the ADCs and the space that’s popping up there and the excitement there. Then you talked about selinexor, which is a completely different mechanism. Can you describe some of the science behind that and why that’s being studied in the maintenance setting?
Dr. Slomovitz: Selinexor (Xpovio) is a nuclear transport inhibitor. It works by keeping good proteins within the nucleus. We did a previous study called SIENDO, which was led by Dr. Ignace Vergote out of Belgium. The SIENDO trial was a similar trial in the maintenance setting to see if selinexor (Xpovio) could improve overall survival and progression-free survival.

What we found is that in the entire population, what we call the intent-to-treat population, it was a negative trial. But in the subgroup of patients that were p53 wild type or normal expression of p53, there was a tremendous benefit of treating with selinexor. Because that wasn’t the primary objective of the study, now we’re doing the follow-up study called the XPORT trial, which is just in the p53 wild type patients, to see if that translates into a better outcome and perhaps FDA approval.
Stephanie: Amazing. And how many people are in the p53 wild type category?
Dr. Slomovitz: p53 wild type is probably about 60 to 70% of endometrial cancers. A lot of the p53s are early stage and don’t need systemic therapy, but we do see still about 40 to 50% of endometrial cancers that are p53 wild type.

Stephanie: Are there any other ones that were missing where you’re like, “This is going to be changing the game for how we present options to certain groups”?
Dr. Slomovitz: I love the way you said that because we’re always looking for game changers. The next strategy is to look at post. We’ve already looked at post-platinum with immunotherapy, post-platinum and post-immunotherapy with ADCs, so the next logical progression is what we do after ADCs.
Hormonal therapy, something called CDK4/6 inhibitors or mTOR inhibitors, is an area that had a lot of previous work in. There are CDK2 inhibitors and Wee1 inhibitors that work particularly against some of these high-risk histologies. There’s another agent, a CHK1/2 inhibitor from Acrivon Therapeutics that’s looking for a specific biomarker signature and if those patients have that signature, they’re being investigated.
There’s a nutritional study looking at PIKTOR (PI3 kinase mutation and mTOR mutation) with chemotherapy and a low-carb diet with diet modifications. The sponsor is Faeth Therapeutics. It’s a cool study — knowing that endometrial cancer is also a product of a high carbohydrate appetite — to see if we could affect the nutritional status.

There’s a lot going on. We have biomarker-driven therapies. There are PI3 kinase mutations that we’re doing. Dr. Stéphanie Gaillard from Johns Hopkins is leading a trial there. There are other antibody-drug conjugates in the second line that we’re investigating.
I talked about first-line treatment. I should mention that the second line antibody-drug conjugate for HER2 has FDA approval already, which is trastuzumab deruxtecan (Enhertu). But we’re looking at antibody-drug conjugates against alpha folate receptor and against B7-H4. There’s a study from AstraZeneca looking at their B7-H4 antibody-drug conjugate. There are a lot of TROP-2s that I already mentioned. There are a lot of good options.

Dispelling Myths About Clinical Trials
Stephanie: A big part of this, Dr. Slomovitz, is translating the importance of clinical trials to people who are just hearing it for the first time from you. I know that we’ve covered some of the questions that people can ask, but what are other ways that you’ve successfully been able to describe clinical trials as an option? Are there any myths you have to commonly bust when they ask about clinical trials?
Dr. Slomovitz: I love the way you said that — clinical trials as an option. I’m preparing a talk that I’m giving to one of the national organizations and that’s what it’s titled: “Clinical Trials as a Care Option for a Patient.” There are a lot of biases against clinical trials. Patients are afraid of them. We need to help overcome those biases.
We need to educate patients that randomized phase 3 trials aren’t placebo-based. They’re giving the current best standard of care versus another drug. Maybe it’s better to get onto the standard of care arm because maybe that’s not as good as the control arms.

We need to balance out socioeconomic imbalances. For example, we need to come up with better support services for patients who don’t have the money to take time off from work, who don’t have the money to park in the parking lot, or who don’t have the technology to do web-based quality-of-life assessments. There’s more and more support from our industry partners. We need to help support the caregiver who brings the patient in. These are important.
We talk to our patients and explain to them that there are no biases and no secrets. We’re trying to get better treatment options for our patients. These aren’t unwarranted biases. Historically, there were a lot of biases towards different populations of patients, and we need to overcome that perception.

Improving Access to Clinical Trials
Stephanie: Can you talk more about any guidance you have for fellow providers on the best way to make sure that we’re not missing people based on their profiles, backgrounds, and socioeconomic status? What are the things that you found to be very helpful in conversations with patients?
Dr. Slomovitz: That’s a great point. Make sure you have a good team around you. Make sure you have nurse navigators and physician extenders — whether it be a nurse practitioner or physician assistants — helping the whole process, reconfirming what the doctor is talking to about.
And then, we have face-to-face conversations, telling patients what the data are, why we’re trying to do these studies, why it’s important to enroll patients across diverse populations, and encouraging their participation not just for themselves, but to potentially answer questions that will treat populations as a whole.
We’re getting better. There’s still bias against it, but we’re getting better.
Stephanie: Good. Especially what you said about Black women dying at a rate two times higher than peers, which is probably due to different reasons. You have groups — I’ll give a shout out to ECANA — that specifically dedicate their advocacy efforts to helping Black women feel connected and get empowered, so I appreciate that.

Understanding the Clinical Trial Process
Stephanie: A simple question, Dr. Slomovitz. When people think of clinical trials, the first time I heard it, I thought, “I’m going somewhere I’m not familiar with. I just don’t have the information,” so I start to write my own story about what it is. What does it actually mean, on a human level, to be part of a clinical trial? I know that there are different considerations depending on the trial, but can you give an example of what patients are going through as they start a clinical trial? What does it look like?
Dr. Slomovitz: With anything, the hardest thing is the unknown, not knowing what to expect. There are a lot of things about clinical research. In addition to the drug that helps the patient get through the process, there’s the clinical team and the research team involved with their care. There are some frustrations in meeting the inclusion criteria calendar. But that’s spelled out and we need to do that. Sometimes, it takes a little bit more time and a few more forms to fill out.
But again, there’s the opportunity to get a treatment that they wouldn’t normally get. There is a great amount of unknown and it’s unknown not only for the patients, but for their family members as well. It’s about fostering an environment of trust, transparency, empathy, and education in order to help overcome some of those barriers. It’s not just saying to the patient, “Oh, don’t worry, we got you.” We have to explain to the patients what we’re doing, why we’re doing it, what the need is, why we are going through extra procedures, and things like that.

Stephanie: People don’t think of the major paperwork. I’m signing my life away. How often do I have to go to the site? Do I communicate with my regular team? Is this a brand new team I’m working with? Those are some common concerns.
Dr. Slomovitz: There’s always the healthcare provider. I’m not the only one at my site who offers the trials that I’m running. The patients stay with their doctor. There may be some extra tests. We like to explain to the patient what we’re doing and why we’re doing it.
I’m a big clinical trial advocate, but in truth, there are some patients who don’t belong in clinical trials. They don’t want the unknown. They don’t want to grasp the understanding that we’re giving everyone the best standard of care. And you can only go so far.
The consenting process is also important, which includes the paperwork that you’re alluding to. Sometimes it can be long. They have to be written on a fifth grade level of understanding. Obviously, a lot of our patients are better than that. But there’s the understanding through the consenting process and through the clinical trials that they don’t need to do it. No one’s ever forced to do it, which is exciting and nice.

How Can I Research Clinical Trials?
Stephanie: We have a great site called ClinicalTrials.gov. They had a refresh a little bit ago, but it’s still not the easiest or most user-friendly site to navigate. Do you have any tips for people? We will list the clinical trials that you mentioned and provide names and links. But for people who are trying to do general research on clinical trials, do you have any tips on where to go?
Dr. Slomovitz: Talk to your doctors. If it’s not a busy center, reach out to providers and other centers. Use the resources, like ClinicalTrials.gov, the American Cancer Society, and other local advocacy and support groups.
I work with the International Gynecologic Cancer Society. We initiated Endometrial Cancer Awareness Month two years ago. It’s in June and peach is the color of endometrial cancer awareness. There’s a great worldwide advocacy outreach globally, which is something that we’re working on.
It’s a matter of digging in deep, doing some work, and getting the information you need. Ask your healthcare providers. Ask your healthcare extenders, nurses, and nurse practitioners, and get the information you need to find out where the best trials are for you. Check with the clinical trial sponsors. I get referred a fair number of patients from companies.

Educating More People About Endometrial Cancer
Stephanie: You were talking about patient-facing efforts. I saw Jump for June as one of those efforts, which is cool. Do you want to share a little bit about that? I know we’ve passed June, but just so people know what it’s all about.
Dr. Slomovitz: As an aside, the CEO of IGCS, Mary Eiken, is a good friend and we’re both Florida Panthers fans. We did the Jump for June. It’s actually on the website. We were both wearing our jerseys when we jumped. After the Florida Panthers won the Stanley Cup, then we got it out there because it looked pretty cool.
Jump for June is a way to raise awareness. You can’t always say, “Watch out for post-menopausal bleeding and abnormalities and go to a clinical trial.” It’s Jump for June. Think about endometrial cancer. What do you need to do to learn more about it? A lot of times, the scariness of the diagnosis and going through the clinical trials is minimized when you’re doing it with someone else. Things like that are important when we talk about public awareness.
Increasing Awareness of Clinical Trials
Stephanie: We’ve talked about clinical trials and how there’s a difference in access when you talk about certain places, like in the community. What is your message to patients and doctors who are in the community setting on what can they do to bring more awareness about clinical trials?
Dr. Slomovitz: We want to try to get as many centers involved with the research as possible. That’s not always possible. Referring their patients to colleagues who are doing the trials, even if it’s just an FYI, to see if there’s anything there or talking to other doctors and going to educational programs where we talk about this. We do a lot of educational work through the GOG and other avenues. There’s not a single area to get the information. There are a lot of different areas.

Final Message of Hope for Patients
Stephanie: For our patients and care partners dealing with endometrial cancer, especially advanced or recurrent, what is your overall message on the landscape of treatment options and what you see on the horizon with all this development?
Dr. Slomovitz: The future’s bright. We’re becoming better and better with our treatment options. It used to be that women with endometrial cancer would get one or two lines of therapy, and then the disease becomes so aggressive that they’d succumb from their disease. Now patients are getting three, four, even five lines of treatment, and as we move forward, we’ll get more and more lines with the latest and greatest therapies.
The two biggest things that we’ve learned is immunotherapy for all patients at some level of their care and these molecular subclassifications helped us to delineate that not all endometrial cancer is the same. And different cancers require different treatments.

Conclusion
Stephanie: Thank you so much, Dr. Slomovitz, for your time. I enjoyed our conversation.
Dr. Slomovitz: Likewise. Thank you.
Stephanie: We encourage patients and care partners to stay informed, ask questions, and partner with their healthcare team to explore all available options.
We would like to thank again our sponsor, Karyopharm, for its support of this patient program. We want to note that The Patient Story retains full editorial control over our content.
While we hope that this was helpful and that you walk away feeling more empowered, please keep in mind that this is not meant to be a substitute for medical advice, so still consult with your own healthcare team about your decisions.
Your voice is truly important to us at The Patient Story. Share with us what we could do better, what you enjoyed, and what you want more of. We’re glad you could make it and we hope it was helpful. See you at a future program. Take good care.
This program has been edited for clarity and length. This is not medical advice. Please consult with your healthcare provider to make informed treatment decisions.
The views and opinions expressed in this interview do not necessarily reflect those of The Patient Story.