The Doctor Talk: Latest Relapsed Refractory Multiple Myeloma Treatments
Dr. Cristina Gasparetto, Duke
Dr. Joshua Richter, Mount Sinai
The list of options of treatments for relapsed refractory multiple myeloma patients and their caregivers continues to grow, so much so that it can get pretty tough to keep track of every possibility out there.
To help sort through the newest drugs and combinations, how they work, side effects and managing through them, The Patient Story hosted top myeloma specialists in our latest event focused on relapsed refractory treatment options:
- Dr. Cristina Gasparetto, Dir. of Multiple Myeloma, Duke
- Dr. Joshua Richter, Dir. of Multiple Myeloma, Blavatnik Family-Chelsea Medical Center, Mt. Sinai
- Introductions
- COVID-19
- Relapsed refractory multiple myeloma treatments
- When do you restart treatment?
- Using more aggressive treatment upfront
- What is selinexor and how does it work?
- Selinexor’s 1st approval
- STOMP clinical trial
- Selinexor side effects and how to manage them
- Isatuximab (SARCLISA)
- Melphalan flufenamide "Melflufen" (PEPAXTO)
- Belantamab mafodotin (BLENREP)
- DREAMM Clinical Studies
- CAR T Cell Therapy
- Bispecific T Cell Engagers (bispecifics)
- Elranatamab clinical trial response
- The bulk of myeloma patients are treated in community setting
- Different bispecifics with different targets
- Pros & Cons: CAR T vs. Bispecifics
- What does it mean to be offered clinical trial participation?
Thanks to Karyopharm for its support of our educational program. The transcript has been edited lightly for clarity.
Transcript
Introductions
Stephanie, The Patient Story: Hi, everyone. Good morning, good afternoon to you, depending on where you’re joining us from. I’m Stephanie with The Patient Story and incredibly excited to lead this program today with our two incredible guests. I’ll get to that in just one moment. Our topic today is treatment options for relapsed refractory multiple myeloma. And as we know, it’s a landscape that is constantly shifting, which is great news.
But that also means it’s a conversation I think that we should continue to have pretty frequently as the information keeps coming in. First, we’d like to give special thanks to Karyopharm for its support of our educational program today. Now, what I hope people you all take away from today’s conversation is more humanized sort of context of all this information that’s coming out. We’re going to have the medical jargon. Of course, it’s part of the conversation, but we’re going to have an incredible discussion that’s led by two top myeloma specialists.
We have Dr. Cristina Gasparetto, Director of the Multiple Myeloma program at Duke University. And we also have Dr. Joshua Richter, who’s the Director of Multiple Myeloma at the Blavatnik Family- Chelsea Medical Center at Mount Sinai. So welcome to you both.

Dr. Cristina Gasparetto: Thank you. Thank you. Pleasure to be here with you guys.
Dr. Cristina Gasparetto: I knew since I was very young, that I was going to become a doctor because my dad was a physician, and his father and his brother and everybody, so it is a long history. And of course, I wanted to emulate my dad.
But myeloma, I was in the first year of fellowship and just my first month rotation. I didn’t know anything about blood cancer, very little coming up on residency. And I was called for a consult, patients with newly diagnosed myeloma.
I tell you the truth that I really didn’t know anything about it. So I went to the library. Back then, because I’m quite old, we didn’t have Google and everything and I read about myeloma and the average age was about 70.

And I went to meet with this patient. And it was a young woman, 42 years old, and she had actually spent 20 years of her life in Italy. So we were conversing in Italian. I’m Italian, and I was fascinated by that, by the story. And I started to read everything and to make a proper recommendation at that time.
And do you know, at that time the autologous transplant was definitely increasing in terms of becoming almost a standard of care and allogeneic transplant. We didn’t have anything. We didn’t have the new agents.
And from that, it became a love hate affair, I guess, with this disease.
Dr. Joshua Richter: I’m the first doctor in my family. I was kind of drawn to this kind of romantic, noble notion of being a physician to help patients.
And I knew I wanted to do oncology hematology, but wasn’t sure about surgical or the medical side until I scrubbed into my first Whipple procedure in medical school.
Well, then, after 14 hours of holding retractors, I realized this is not for me. And then I did my internship in residency at a hospital that no longer exists called St. Vincent’s. And it was an epicenter of myeloma with people like Sundar Jagannath, Ajai Chari, David Vesole, Madhav Dhodapkar.
And my first day on the oncology ward, when I get up to the floor to see the admitted patients and understanding that myeloma is less than two percent of all cancer, the hospital is very known for HIV and myeloma.
And normally an inpatient floor will be lung cancer, colon cancer. But because it was HIV, the first three patients had Burkitt’s lymphoma, another rare disease, and every other bed was myeloma, which is just a very strange thing for an oncology floor.
And I got to see world class people treating a complex disease. And I find that fascinating that the field was in this earlier phase of evolving exactly as Dr. Gasparetto talked about.
The older days, there was little we could do. In the last 10, 15 years have been an explosion of options. And it just seemed like a place where you can make a huge impact. So I got hooked.

COVID-19
What is your guidance on the third vaccine shot?
The Patient Story: I’d like to kick off with some COVID-related questions, actually, just because it’s timely. You know, people are talking about with the CDC’s recommendation about hey, you’ve got to go get your booster shot, your third shot. For a lot of folks, they’re hearing more people talk about certain things.
So they want some clarification and we’re hoping that maybe you can provide some. One of the questions is about how some of the treatment immunosuppressive, might prevent a better antibody response from the vaccines. How bad is it?
Dr. Cristina Gasparetto: We don’t have a lot of data on safety but it looks like a lot of patients with myeloma already participated, they are participating. And the problem is that we don’t know, we don’t know if the third boost is going to protect them even more if they are taking special treatment.
When I talk with my patients, I always stress the importance of maintaining precautions – the masks, the social distance, and then at the first exposure, I think if they are exposed, very quickly to contact us to receive antibody treatment.
So we actually have at Duke infectious disease, we can move very fast on providing them with the infusion of the antibody infusions to minimize the risk of major symptoms with infection. So we don’t know. We’re learning about it and I think the Leukemia & Lymphoma Society are going to collect the data about the third shot, the boost.
And right now, do you start it? So we have some recommendations and we can provide recommendations to our patients in active treatment less than two years from transplant to go ahead with another booster. But we don’t have a lot of data on safety.

Dr. Joshua Richter: A third booster may help some people, but there may be people whose immune system is so far down that if two didn’t do it, a third won’t do it. I think the best advice is exactly what Dr. Gasparetto said, which is until we are on the other side of these big waves, and we learn a little bit more, continue masking and social distancing.
What precautions should myeloma patients take in spending time with unvaccinated kids?
The Patient Story: Perfect, the message being, of course, you know, better to get the booster shot, especially if it’s been effective than to not, and then just take those extra precautions.
A second question comes from Jane L. who says her father was recently diagnosed with multiple myeloma. She’s worried because he spends a lot of time with his grandkids and one of them is six. So unvaccinated and going back to school now, so is masking up enough? Is there any sort of general guidance there? And we can start with you, Dr. Richter.
Dr. Joshua Richter: Yeah. I mean, these are the really, really tough questions that we always have a saying in myeloma: You ask three myeloma doctors a question, you will get five answers. So I don’t know that there’s a clear guideline. The likelihood is that there’s going to be vaccinations for two and up later in the year.
This is a really difficult time, especially with, they talk about this thing called the R nought (R0), which is how many people you’re likely to spread the infection to.
And the original Covid was more like an R0 of two. And now with the Delta variant, it’s like nine. So the concern is as kids get back together after the summer, they’ll share a little bit. Teachers may share and they may bring it back.
So I think the way I would approach it is I think everyone has been very diligent and it’s taken a huge toll on everyone, physically, psychologically. But early on in the next year, where we have more people vaccinated, including people potentially as young as one year old. I think it’s going to be a different story. So I would say still try to keep a little bit of distance.
Relapsed refractory multiple myeloma treatments
The Patient Story: Ok, perfect. Thank you so much for that. So as we shift now into talking about these treatment options and his shifting landscape, we do have to set the stage a little bit. Everyone knows in the space that myeloma, the experience ranges widely.
And Dr. Richter, you just said ask one question, get five answers. So we should expect that and want to remind everyone that today’s discussion is very much a discussion, it’s about this information, empowering patients and caregivers to take what they can from it, but that it is not medical advice.
When do you restart treatment?
So the first one is about deciding when and how to restart treatment. You know, what are those signals that you take in? And I know it varies, again, from patient to patient. And how much does it vary depending on the standard risk versus high risk? And maybe we’ll start with you, Dr. Gasparetto.
Dr. Cristina Gasparetto: Sure, it’s always a very difficult question because you are correct. Standard risk, high risk probably is a little bit different. If you have a patient, for instance, I’m going to give you some examples so it makes more sense.
Standard risk
If you have a patient with standard risk myeloma five years after transplant, remission, and now you check labs and you start to see a resurfacing of the protein marker. Do you need to treat the patient immediately? Probably not.
The first step that we do, we do staging to make sure that the myeloma is not causing any damage. And then we monitor the amount of care that protein and if the protein remains relatively stable.
Sometimes we postpone treatment because we know that as soon as we initiate treatment, we have to go with three drugs, a combination and we increase the risk of toxicity. So we don’t want to do any harm and we want to, you know, if every drug has a half life and the clock starts to tick when you start the drug, if you start the point, you have enough time.
So if you postpone the initiation, it may be beneficial. You may have something else to offer to your patient later on and decrease, improve. And particularly if the patient comes to you and says, I feel okay, I don’t have any symptoms. Why? And to follow that particular patient.
High risk
Now, if a patient progresses more rapidly, if they’re still on treatment or if it’s very quickly after the transplant, or a patient with high risk myeloma,that is a different story. Then we have to take that in account and probably initiate treatment a little bit sooner at that point.
And of course if a patient has symptoms, and we find out that the myeloma is already damaging then yes, absolutely, no question.
Dr. Joshua Richter: Perfect. Yeah, I couldn’t agree more. You know, I think one of the things you brought up, Stephanie, is this notion of high risk or standard risk. And typically when we talk about high risk, we talk about cytogenetic high risk.
So abnormalities in the DNA of the cancer cells, things like deletion of part of the 17th chromosome or extra copies of the first chromosome. But something that Dr. Gasparetto brought up, which is absolutely critical in deciding who needs retreatment, is something we call functional high risk.
So with many of our great drugs, we anticipate that initial response to induction or a transplant to last many, many years. If your disease relapse is much sooner than that, it doesn’t matter if your genetics don’t show any high risk. You’re demonstrating the biology of the disease to be functionally high risk.
So I completely agree with Dr. Gasparetto. We’ve had patients, a few months after the transplant, the numbers are coming up. That’s an omen of something bad. We may have to act on that sooner.
Using more aggressive treatment upfront
The Patient Story: I appreciate you both responding about that. I’d like to ask too now and we’ll start with you, Dr. Gasparetto. It’s generally said that the first line response tends to be deeper, longer than the second. I’m curious, what’s your opinion about starting with more aggressive treatment up front?
Dr. Cristina Gasparetto: Yeah, well, we have a lot of data on the achievement of the deep response, and we have a lot of data showing if a patient achieves that deep response, we probably have a better outcome or remain in remission longer.
It looks like we are trying to go to the same goal, even in second relapse we’re pushing the envelope more and more.
And, you know, actually, when I started, we were not bringing everybody to a complete remission. It was very difficult to explain to patients, because after transplant, some patients who still had residual myeloma, and we were saying, oh, it doesn’t matter. You know we’ll see if the myeloma is resurfacing sooner or not.
But now we’re pushing, we are becoming more and more aggressive like other cancers, right? At the beginning it didn’t make sense because if you treat any other type of cancer, you want to achieve a complete remission.
With myeloma, we were like, it doesn’t matter. But now we know it matters. And we are accumulating more and more data. And I think achieving a deeper response is important. Even in cycle maybe third line, and that correlates with a better outcome.
But what’s happened is sometimes, we can’t. Later on we cannot do that. It’s clear. And in fact, some of the new approaches, what is amazing to us, that we can reach the depth of response. We’ll talk later about the CAR T and other immunotherapy and even in later life of the myeloma. So we are learning about that. But, yes, we keep that as one of the goals earlier on. And then sometimes with time, unfortunately, we may not be able to achieve that.
Dr. Joshua Richter: It’s definitely been this push and pull, as we slowly develop more and more drugs. The question was:
- What’s the best way to use them?
- Do you give just one or two at a time?
This way you have something for later on. Combining different chemo drugs with using classical chemo. So old school chemotherapy drugs like melphalan and etoposide and adriamycin, the older generation of chemo. Combining multiple drugs can be very toxic. But many of the novel or newer drugs we use in myeloma could be combined much easier.
There’s been discussions of do you save things for further down the road or do you make these three and four drug combinations earlier and earlier on? And we now know that that’s probably the better way to go.
And now that we have more drugs, we have three unique drugs to give upfront, three unique drugs to give second, third and fourth line.
It’s really important to put your best foot forward, because despite the probably dozen or more drugs we have, the average patient in the United States only gets three or four total lines of treatment for both good and bad reasons.
The good reasons being that some people do so well with their first or second line. They can live for many, many years, and pass of something else, in complete remission.
Unfortunately, some people have higher risk disease and don’t make it beyond the first few lines because they’re so sick. But yeah, achieving deep remission now with multiple drugs is really key and we know that people get these deep responses, such incredible outcomes.
We used to talk about the entire journey from diagnosis to death being only two years where there was a recent study that looked at giving daratumumab, REVLIMID and dexamethasone to the older, frailer patients who were not eligible for transplant. And they just recently looked at the data.
And the average remission has not yet been reached, but across the five year line. So we’re talking about the older, frailer patients who can’t get transplant with their upfront therapy. What used to be the entire journey from diagnosis to death was two years. Now, the older, sicker patients get more than five years on average with their first line alone.
Dr. Cristina Gasparetto: Absolutely, I think some of these combinations are becoming so powerful and we are learning the early exposure of some of these agents is very important and the combination.
Definitely we’re treating the myeloma very differently from even five, 10 years ago.
The Patient Story: It is so promising and so energizing to hear about even these numbers. It just paints that picture for people. Perfect. Ok, I hear a lot of agreement there between the two of you, that’s wonderful.
Ok, so then without further ado, I think a lot of people are curious about these newer therapies, newer combinations. And in this context, there is a lot of information out there already I’ve seen about what it is and more of the medical jargon. We do have to talk about, you know, things like how it works just so people understand these different classes, they work differently.
But I’d love to get some color from the both of you in terms of your own experience with patients. The real world experience. And we got a lot of audience questions. I’m just going to sort of throw it all in general to say they wanted to know about side effects going through each one. And of course, the most important is how you try to reduce them, if possible.
Selinexor (XPOVIO)
What is selinexor and how does it work?
The Patient Story: So we’ll start with selinexor, trade name XPOVIO, just because it was more recently approved to be used earlier. And so I think there’s a lot of curiosity about that.
That approval was for selinexor, bortezomib or Velcade, and dexamethasone. Dr. Gasparetto, we’ll start with you. Can you talk about how it works and then experience with it?
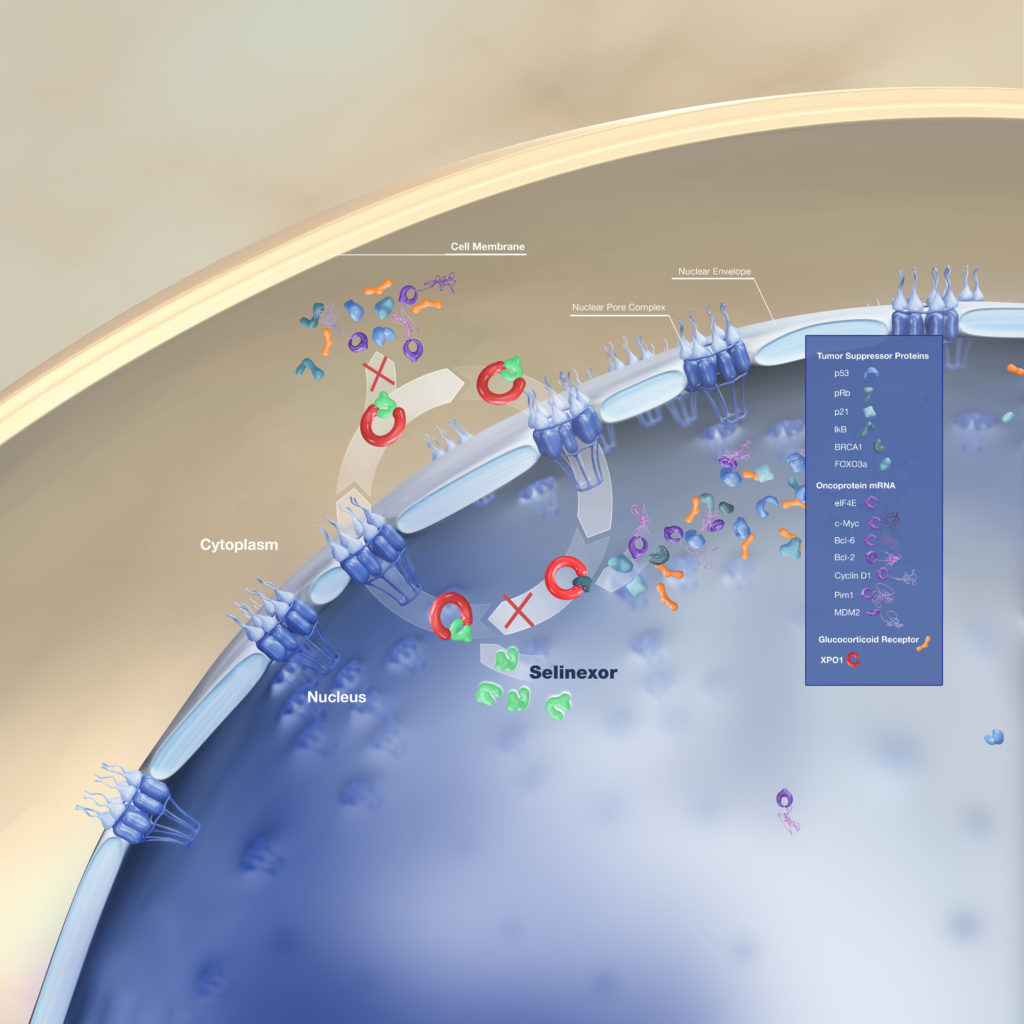
Dr. Cristina Gasparetto: Selinexor, first of all, is another oral, by mouth, medication. So it’s always good to explain that to the patient. So for convenience, who doesn’t like an oral medication?
And it has a different mechanism of action, but it is not unique of myeloma. In fact, XPOVIO has been used for different type of malignancies, including solid tumor.
XPOVIO blocks a very important protein. It’s called exportin-1. And the reason why it’s important for cancer, because keeps the cancer cells going on, multiply and staying alive. And I’m trying to explain these very simplistically.
So XPOVIO blocks this protein. So the cells eventually, because this protein is blocked, they go what we call in apoptosis, they go to death. So they die. And these exportin-1 is over-expressed in myeloma and actually correlates with worse survival or worse prognosis in a way. And so it works very well.
Selinexor’s 1st approval
When XPOVIO was first approved, it was in combination with dexamethasone in heavily-pretreated patients. Joshua was part of the STORM study, XPOVIO was given twice a week to patients that we call refractory to everything, the penta-exposed, so very difficult to treat. And there was a signal.
However, very difficult to tolerate. I think you guys did a lot to work with the supportive care, the triple antiemetics anti-nausea medication to support the patient. And we don’t use XPOVIO like that anymore. That’s the passé, it’s old school.
Selinexor’s latest approval

Now XPOVIO is once a week and when we use once a week in combination, we are able to mitigate some of these side effects. And that’s the reason why XPOVIO was recently approved in combination with bortezomib once a week. And patients are able to tolerate the agent XPOVIO much better.
In fact, we have a lot of data on the nausea, the fatigue that kind of dissipates with time. If patients are able to, I always tell patients that you just have to, in a way, toughen up for the first couple of cycles and then we can maybe be able to adjust the dosage and you’re able to tolerate.
So XPOVIO was approved in combination with dexamethasone twice a week. We don’t use that anymore. We use it in combination.
STOMP clinical trial
(Selinexor and Backbone Treatments of Multiple Myeloma Patients)
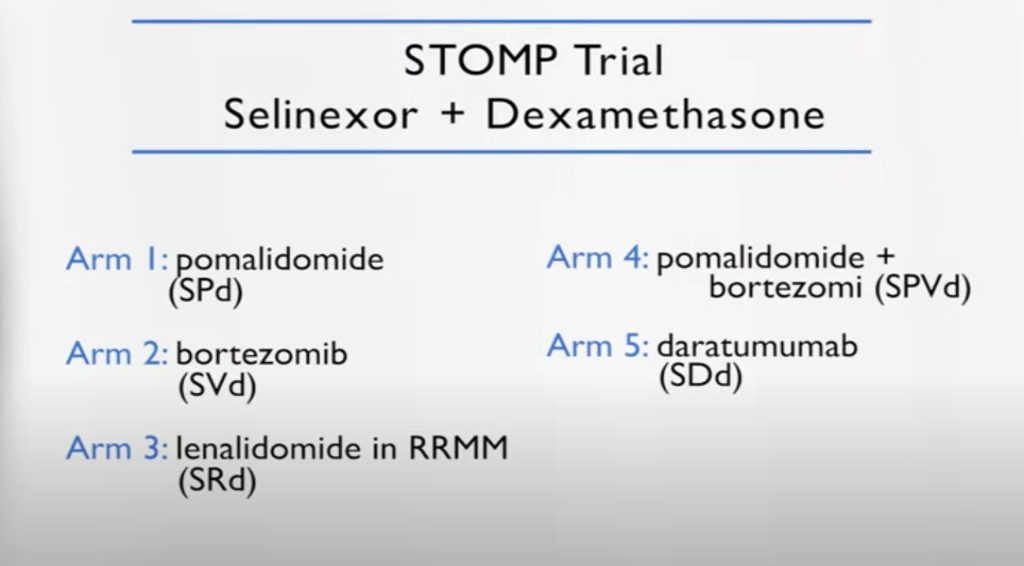

And I’m part of a study called the STOMP study, where XPOVIO is used in combination with everything else. So bortezomib, which led the launch of the BOSTON study and then carfilzomib, daratumumab, REVLIMID, pomalidomide – you name it.
We present, we channel the patient based on what they were exposed to before. So for example, in the carfilzomib XPOVIO they were naïve to carfilzomib and so forth. We discovered during the study that in certain combinations, it’s very effective.
Once a week we adjust the dosage and they’re relatively well-tolerated. In fact, I have patients able to sustain therapy for a few years. And everybody was like, how did you do that?
Because with adjusting the dosage and the proper supportive care at the beginning. It’s definitely a very powerful drug, and we have data on high risk myeloma. We have data that we learned from the BOSTON study on how to manage the toxicity.
So it’s a new drug and it went totally from the very heavily pretreated to the BOSTON in first relapse. There are some very interesting combinations that we launched from this STOMP study. And probably they are going to be becoming more established based on the early results of the STOMP study. So it’s a very interesting drug, it’s a new mechanism of action for myeloma, a new target.
Dr. Joshua Richter: I think this brings up something that’s absolutely crucial is that there have been studies to show that if patients at least see a myeloma specialist like Cristina, myself, or any of the number of places in the country and in the world, you will do better long term because you will take advantage of the expertise of someone like Dr. Gasparetto and be able to take that locally.
And I always talk about myeloma kind of like remember the movie The Matrix? They say some rules can be bent, others can be broken. You know, the more insights you have, the more you can navigate that.
You know, the fact that these regimens, some of the STOMP ones have NCCN guideline approval means that Medicare will cover them, but only if your practice team knows about them and knows exactly what Dr. Gasparetto was talking about – how to dose these drugs, because giving 80 milligrams twice weekly of selinexor is very complicated. And exactly what she said, we don’t do that anymore.
But doses between 60 and 100 once a week, especially with other drugs. So when it was by itself, it had to do all the heavy lifting. When it has other drugs to synergize with, you can bring the dose down, add supportive care and really get these much better outcomes.
Selinexor side effects and how to manage them
Nausea
- dexamethasone
- Zofran
- olanzapine (Zyprexa)
- rolapitant (Varubi)
Dr. Joshua Richter: The way that we really approach this is that old saying an ounce of prevention is a pound of cure and really being very aggressive in the early cycles with trying to prevent toxicity instead of waiting until it shows up, because then nausea is one of those complicated ones once it shows up, patients can then have anticipatory nausea.
So a lot of work was done with one of my colleagues as well, Dr. Ajai Chari, looking into different combinations of drugs to help prevent nausea and some of the standard ones we use, like dexamethasone and Zofran.
But two ones that are a little bit off the beaten path seem to really help. One is a drug called Zyprexa, which is olanzapine, which is a drug that’s been used in the psychiatric world. But it also really is good at preventing nausea.

The other is a drug called Varubi or rolapitant. It’s a very poorly used drug in general. It’s the same class of drugs as things like Emend or aprepitant, but it doesn’t have the same drug interactions with selinexor that Emend might.
So I think that’s one thing I would encourage all patients, even if you love your local team, now especially in the days of COVID, where we have telemedicine, go out of your way to seek care at a place like Duke or Mt. Sinai, or one of the many advocacy groups that can help you get connected with one of the great myeloma centers in the country.
The Patient Story: Absolutely. Myeloma specialists. I mean, we are talking about just how many variations and presentations, and it’s tricky. And so being able to talk to someone who focuses just on this, I think is really important. This is part of why we do these things, to reach people who are in the community setting to help them out and get them going on the right path, hopefully.
I have olanzapine, I think, in my cabinet from when I was treated for the nausea. And it’s exactly right. Right. Like don’t start to chase as soon as you start to chase the nausea, it’s just like it’s impossible. It feels like. But I haven’t heard much about Varubi.
So I was going to ask you about, you know, all the work that you’ve done looking into the side effects and the management. Is there anything else? I mean, I think the nausea is a huge one. And anything else in terms of I know that sometimes they talk about loss of appetite, you know, things like that.
Weight loss
Dr. Joshua Richter: So loss of appetite, you definitely want to talk to someone more like me than Cristina, because she’s in phenomenal shape and you cannot see from here down but I eat a lot more than Cristina does. So I have plenty of tips for people to gain weight. One of them is really being ahead of the game again, helping prevent it. Small, frequent meals.

Loss of or change in taste
- lemon juice
- sour candies
- Carnation instant breakfast mix
The other thing is sometimes with chemotherapy, people’s taste may go off because the taste buds are not high on the pecking order for the body when they’re dealing with other things. So food often tastes bland or like paper.
Sometimes a little simple thing is taking that lemon juice bottle that looks like a plastic lemon we all have in our refrigerator for 100 years, squirting a little bit of that on the tongue or sucking on some sour candies prior to a meal may liven up the taste buds to help food taste better for people who are losing weight.
Remember Carnation Instant Breakfast? You can use Carnation Instant breakfast, but instead of mixing it with milk, mix it with ice cream so that even if you’re only taking a few small bites of food, each bite is very calorie dense.
Hypernatremia (low salt levels in blood)
The other side effect is selinexor can cause a little bit of what we call hypernatremia, low salt levels in the blood. Usually not very symptomatic, but again, recommending things like potato chips and pretzels can help along with that.
Blood platelet counts
The other thing is it can affect the blood counts, specifically selinexor seems to affect the platelets.People may get things like growth factors throughout their treatment course and people may be more familiar with drugs like Procrit or Retacrit which raises the red count or Zarxio or Neupogen (filgrastim) that raises the white count.
But there are drugs that raise the platelet count. The two big ones are eltrombopag or Promacta and romiplostim or Nplate. And our experience is that you need to titrate the doses of that up quickly and pretty high to overcome some of the low platelet count you’ll get with selinexor. But again, we see that more when we use selinexor. Towards the very end, less of it when we use it earlier on.
The Patient Story: Perfect. Thank you so much. I know that was a lot to get through, and I think that’ll be incredibly helpful for people – start Googling things. I’d like to shift over now to isatuximab or Sarclisa, a monoclonal antibody. We will start with you, Dr. Richter, if you want to talk about how it works and your experience with it.
Isatuximab (SARCLISA)
What is isatuximab and how does it work?
Dr. Joshua Richter: All right, so SARCLISA or isatuximab is a monoclonal antibody, and just like our body makes antibodies to fight infections, this is an engineered antibody to attack myeloma. All cells in the body have different markers on them to kind of interact with other cells and take out various actions.
And they all go by these names CD – CD1, CD2, CD583. CD just stands for cluster of differentiation. All cells have different groups. So lymphomas have CD20 and CD3. Myeloma cells universally have CD38.
This is a naked antibody, which means it doesn’t come along with any toxin or anything. It’s just the antibody. It seeks out anything that has that CD38 marker, grabs on to it, and can induce that cell to undergo what Cristina talked about earlier, apoptosis, through a number of different mechanisms, complement-dependent cytotoxicity, antibody directed. There’s a few different ways you can do it, and it directly kills the myeloma cells.
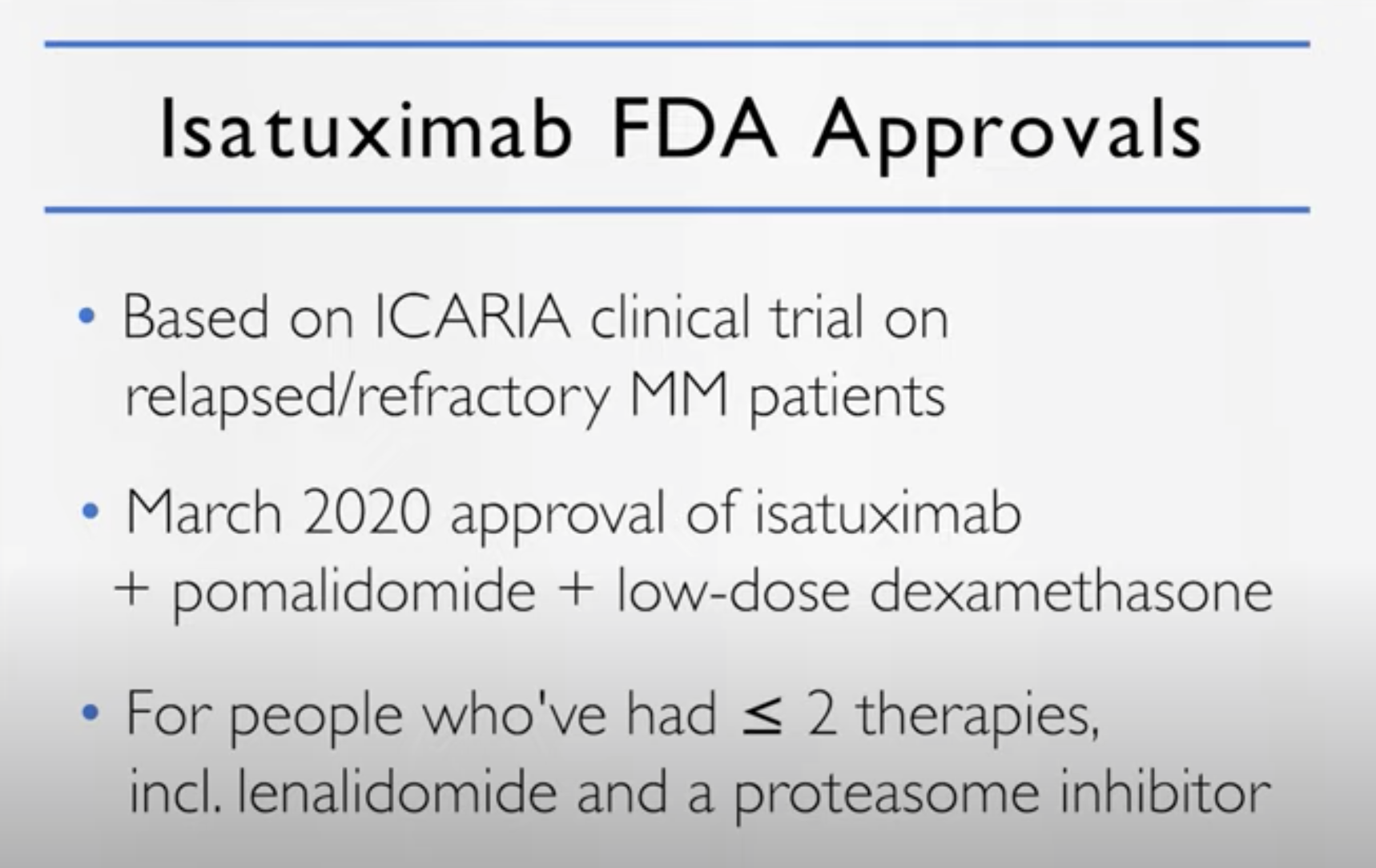
Current isatuximab FDA approvals
Isatuximab currently has two FDA approvals, one based on a trial called the ICARIA trial, which gave isatuximab, pomalidomide and dexamethasone to refractory patients, and the IKEMA trial more recently, isatuximab, Kyprolis and dexamethasone.
Although many people may be more familiar with daratumumab, which comes now in both intravenous and subcutaneous administration, isatuximab is only intravenous at the moment. There are ongoing studies with the subcutaneous version.
Isatuximab side effects & how to manage them
The biggest side effect is that the first dose can have an infusion related reaction, whereas as the drug is going into you, you can get shakes, chills, and flushing. We give medicines ahead of time to prevent that.
If you do get it, the nurses will have a protocol to give medicines to stop it immediately. And generally, after you get beyond the first or second dose, most people don’t have any side effects and is overall a very well tolerated drug and is definitely part of our treatment paradigm for relapsed and refractory myeloma.
Dr. Cristina Gasparetto: I’m jumping from one thing to the next. Over the next few years, hopefully soon that we are going to adjust tailor our treatment based on the patient, the age, comorbidity, genetics, type of myeloma. And so we are learning.
Our job as a myeloma expert is to learn the difference, the nuances between one regimen and the next. Am I going to choose isatuximab carfilzomib versus isatuximab pomalidomide based on the age again? And so relatively well tolerated in the elderly with the carfilzomib.So we are dissecting all this data. There is no doubt that these regimens are very effective. Now, the question for us is,sequencing.
Preference of how drugs are given
The Patient Story: I love all of that. And the goal, of course, the trend of more precision medicine and tailoring.Incredibly exciting. Another factor and you both brought it up. I mean, Dr. Gasparetto you talked about selinexor being the oral pill. Who doesn’t like just popping a pill?
And Dr. Richter, you also we were talking about how isatuximab right now it’s IV only. Sometimes there’s some reaction to that, whereas daratumumab, there is that shot. How often in your conversations with patients is that coming up in terms of how it’s given? So let’s not talk about the pill. I think that’s always pretty preferred. But the shot versus the IV, does that come up?
Dr. Cristina Gasparetto: Of course. Of course. In fact, one of the convenience, you know, the daratumumab with pomalidomide, when you go to dara monthly with pomalidomide becomes very convenient.
But a patient with high risk myeloma may in fact, benefit from the IV infusion and the consistency of the IV, the drug, with a different mechanism of action. So while we are talking about convenience, we are also talking about, you know, this is probably a better regimen for you or vice versa. So it’s part of the discussion, of course.
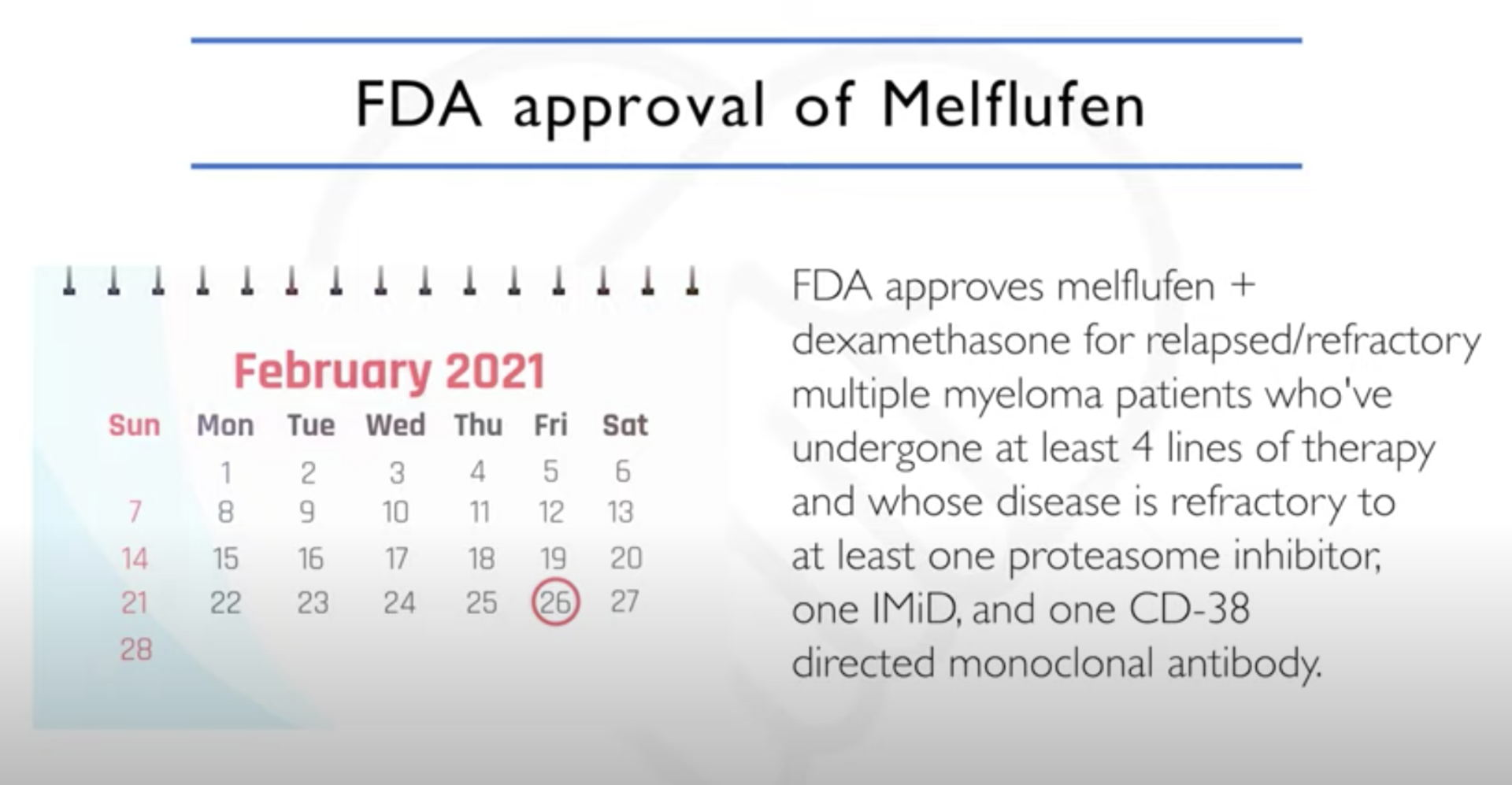
Melphalan flufenamide “Melflufen” (PEPAXTO)
What is melflufen and how does it work?
The Patient Story: Perfect. Wonderful. We will shift over now to melflufen, trade name PEPAXTO, and this is maybe a little bit later on for the patients who’ve had four or more lines of prior therapy. Let’s go to you, Dr. Richter, to talk about melflufen and your experience with it.
Dr. Joshua Richter: Sure. So, melflufen tries to marry one of the greatest drugs in myeloma history to the new way. So when we talk about chemotherapy or drugs, there’s kind of a dividing line.
There’s classical chemo that was invented around World War 2 that just kind of kills everything. And then there’s the newer therapies that better target the bad cells and less off target toxicity. Melphalan is the drug that has been given both orally and the majority of the way we use it in the US is to give high doses of melphalan as part of our stem cell transplant procedure.
What melflufen is is it’s a peptide driven melphalan and which basically because the peptidases are overexpressed in the cancer cells, the cancer cells will suck this up more than normal cells. And it delivers the melphalan more specifically to the cancer cells than to others. So think of it as a newer way of giving melphalan.
How is melflufen administered?
Dr. Joshua Richter: It’s a very interesting drug. It’s given once a month. So it’s nice to give a drug only once a month. It is an IV. However, at the current time, it requires a central line.
So a central line can either be a triple-lumen catheter or a PICC line or any other way to get central access. There is an ongoing study called the PORT study, which is evaluating giving the drugs for a peripheral IV, which will hopefully be out later this year, which will allow us to give the drug through a peripheral IV instead of a central line.

Melflufen’s status (October 2021)
Dr. Joshua Richter: I’ve used the drug with some success. I think it’s a very powerful drug. At this very moment right now, there are a lot of issues at the FDA level. In July of this year, there was an independent review committee that looked at another study that was done that was head to head, melflufen versus pomalidomide.
And even though the progression free survival was still better with melflufen, patients stayed in remission longer, the overall survival was shorter with melflufen. So the FDA put a bit of a hold at the moment.The FDA and the company that makes this, Oncopeptides, are currently going through discussions of trying to tease out who was having the poor outcomes, who are having the better ones. So the future is a little complicated.
Melflufen side effects & how to manage them
Including, not limited to:
- low blood counts
- low platelet counts
Dr. Joshua Richter: The biggest side effect is it lowers the blood counts. Apart from cytopenias or low counts there are very few other toxicities, specifically thrombocytopenia and low platelet counts. So the patients that I have, I usually have to delay their next cycle a little bit because their platelets haven’t quite come up just yet.
And there’s some really great ongoing studies in the same vein of Cristina’s STOMP study of combining it with drugs already out there and the two most mature datasets are melflufen plus bortezomib, and melflufen plus daratumumab.
The good thing is in the study, that allowed the FDA to approve melflufen in combination with dexamethasone. There was a group of patients who had what we called extra medullary disease, meaning that the myeloma was kind of losing control a little bit and presenting with several tumor outside the bonds.
And there was a good signal. So it’s old fashioned chemotherapy, but it’s more targeted than chemotherapy. So we learned very quickly that can be used in these difficult to treat population of patients with extra medullary disease. And that was a good signal, a good response rate.
The Patient Story: It’s all promising. What I’m hearing, of course, is we’re in the thick of things and all these different combinations. People are trying to tease out what is that right combination. Different people respond differently. I also like that you’re talking about, before you talked about different doses of selinexor for instance, that can help with side effects and you can play around after a couple of cycles. People tough it out. And then, Dr. Richter, you are talking this time about the schedule, right. So maybe you will have to delay for a few days so that it’s more palatable.
Dr. Joshua Richter: Maybe dose reduction in combination. We are, I guess, establishing the right dose in different combinations, and it may not be 40, maybe 30 or lower.
Belantamab mafodotin (BLENREP)
Belantamab mafodotin and how it works
The Patient Story: Perfect. Great. And so our last one for this section is the belantamab mafodotin or trade name BLENREP, which again is a little bit later, the FDA approval is for 4+ prior lines of therapy. Dr. Gasparetto, could you talk about how it works and again, your experience with it, with your patients?
Dr. Cristina Gasparetto: We call it a conjugated antibody. It’s like Joshua was explaining about the daratumumab. So the target now is different, it is not CD38, but it’s BCMA B-cell maturation antigen, which is becoming a very important target for myeloma, a new one. And so it’s conjugated, these are linked to a toxin, the mafodotin.
It kind of binds to the myeloma cells, the BCMAs mainly expressed on the myeloma and mature B cell. Then there is the delivery of these microtubule toxins. And so that is, again, the destruction of the cells, the cells’ death.
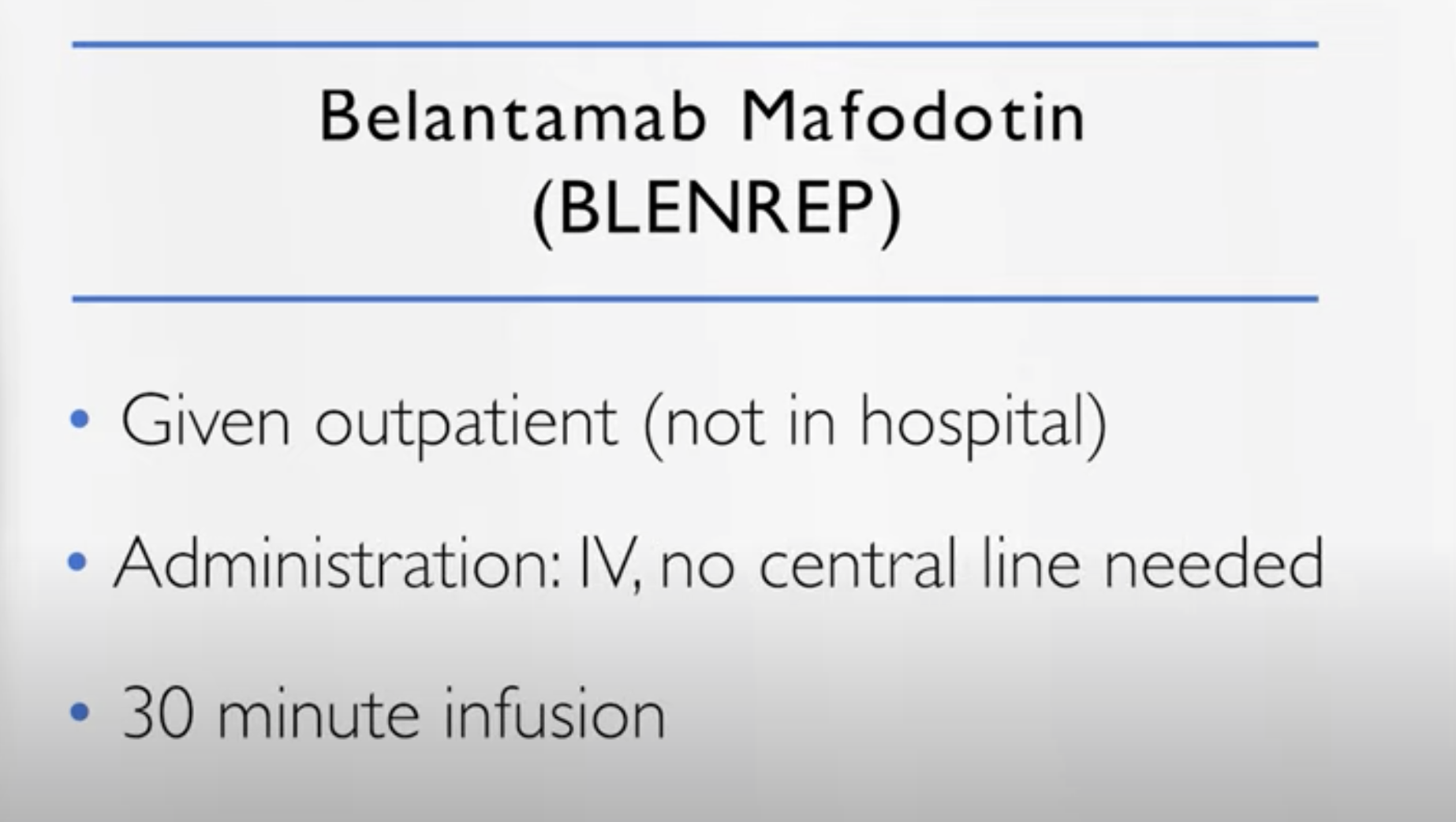
How is belantamab mafodotin administered
Dr. Cristina Gasparetto: So this drug is very convenient, is given in the outpatient setting. IV, but you don’t need a central line, it’s about a 30 minute infusion. It comes, though, with the big warning. The ocular, the eye, toxicity.
The drug is a single agent. Doesn’t have dexamethasone, doesn’t have steroids. It works. And some of the responses are very durable.
Even when patients are skipping the dosage because of the toxicity. But you really have to sit down with a patient in explaining what the ocular the eye toxicity involved, what it is.
Belantamab mafodotin’s biggest side effect: Keratopathy
Dr. Cristina Gasparetto: Patients can have what we call keratopathy, which is the microcyst formation of the corneal epithelium. And at the beginning they don’t have any symptoms. 50% of patients don’t have any symptoms.
So the importance is to establish a partnership with the eye care, ophthalmology, optometrist. So patients are followed by both of us, by the eye ophthalmologist, optometrist so we can decide together if we can continue.
We can give the infusion every three weeks, because all these ocular toxicities in the clinical study were reversible because people would present with this keratopathy, decrease of the eye, the vision, blurred vision, dry eyes, and they are reversible.
As long as you don’t continue on the infusion, you don’t want to arrive at the severe keratopathy, the grade 4 with the ulceration. You want to stop it. And so the partnership and explaining to the patient that, of course, of these ocular toxicities is reversible.
They’re not going to get blind, but when we’re dealing with eye toxicity, we all get scared, and unfortunately it’s the deliveries, the toxin that is linked to BCMA, to the antibody that is causing directly that.
How can patients manage the keratopathy
Dr. Cristina Gasparetto:
A lot of studies are trying to prevent, trying to help patients, lubrication of the eye. Don’t use the contact lens and keeping a constant, you know, follow up with us, with the physician and the ophthalmologist or optometrist so we can guide. And the beauty of this drug is there are some patients who just skip one, two, three doses. And the responses were not changing. They were able to maintain their response.
I have patients that are continuing therapy and doing quite well. And I’m impressed by the durability of the responses. There is a lot of press and talk about the ocular toxicity. But when you are counseling the patient and explaining that we prevent, we don’t want the patient to develop the severe toxicity and they’re reversible, I think it is a very good drug.
And we’ll see the evolution also this drug in other combinations. But so far, I’m quite impressed with the drug and the efficacy of this drug.
Dr. Joshua Richter: I couldn’t agree more. It’s definitely an important tool that we have, and the really interesting thing about the drug is, Stephanie, you mentioned,in terms of melflufen, sometimes a dose reduction is going from 40 milligrams down to 30, but sometimes it’s going from giving it every four weeks to every five or six weeks.
Same thing with Velcade. When you have 1.3 as the starting dose twice weekly, you can go to one twice weekly or one point three once a week.
Belantamab dose schedule

Dr. Joshua Richter: And with belantamab, we have those options, the starting dose is 2.5. We can go down to 1.9, but probably the better way to dose reduce is to stretch out the frequency of dosing.
So it’s prescribed as an every three week drug. But my experience has been it’s mostly an every four to six week drug because you kind of have to space it out.
And that’s a dose reduction simply by reducing the frequency and the benefit of extending it out to every four weeks or every eight weeks is it starts to kind of jive better in combination, because most of our other drugs, like daratumumab, like Kyprolis, like the IMiDs, are on 28 day cycles.
So it’s probably better as a every four week drug. And earlier on, maybe in first or second relapse, some of those trials or even giving it every eight weeks. So basically once every other four week cycle when you’re giving it in combination. And the same thing there, you know, we may have to use a different drug.
DREAMM Clinical Studies
So all of the studies that are being done with belantamab are called DREAMM with two M’s. So the drug got approved by the DREAMM-2 study. The most robust data so far [00:59:30] that I’ve seen is from the DREAMM-6 study, which is mostly being led by Dr. Ajay Nooka at Winship at Emory. And it seems right now that when you give it with Velcade, it’s probably 1.9 is the dose.
So we learn so much more about these drugs after they’re approved, what the right supportive meds, what the right dosing levels, schedule. We all learn this as we use the drug.
Dr. Cristina Gasparetto: I agree. Also with a pomalidomide was presented with a longer interval. And those are new.
The Patient Story: It’s interesting to hear all these descriptions, and as you said, everyone has to stay tuned to see how the trials progress and then you get more data and know how to apply that to people, which is what we are all waiting for. But as Dr. Gasparetto was saying, when you talk about eyes, you know, it’s just..
Dr. Cristina Gasparetto: It is intimidating, right?
The Patient Story: Yeah, very intimidating. And I think, you know, people might have heard the term REMS, but it’s the risk evaluation and mitigation strategy. But really, you were both talking about, you know, you just have to have more of the follow up you have for safety.
Dr. Cristina Gasparetto: For safety. Absolutely.
CAR T Cell Therapy
Idecabtagene vicleucel or ide-cel (ABECMA)
The Patient Story: So that’s great to know. And now I’d love to shift over to a very exciting, of course, space. You talk about CAR T cell therapy and bispecific T cell engagers or bispecifics for short for now. So I’d like to set up first with CAR T.
What is ide-cel and how does it work?
I know that we’ve got ide-cel or ABECMA that’s been approved. Would love to know your experience with that. And I know there’s also a lot of excitement coming up with cilta-cel, which is expected to be approved hopefully shortly. So we’ll start with you, Dr. Richter.
Dr. Joshua Richter: Sure. So the old way of cancer treatment was just giving poisons to poison the bad cells and recognizing that along the way you would unfortunately poison some good cells.
What has really kind of evolved over the last period of time is this concept of immunotherapy or taking advantage of the fact that the best cancer fighter that exists is our own immune system. It fights cancer 24 hours a day.
The difficulty is that sometimes in an individual, parts of the system may break down. And what CAR T does is it tries to kind of rev it back up by collecting the T cells of a patient. And for many patients who’ve been treated, they may have gone through a similar experience collecting stem cells for transplant.
You need to get a catheter. You need to have your cells extracted on an apheresis machine. But the dial is switched. Instead of collecting stem cells, we collect T cells, which are some of the immune cells. In the lab, they’re engineered to all attack a specific target that’s on myeloma.
Ide-cel’s target
Dr. Joshua Richter: And right now, the main target is this BCMA, the same target for BLENREP, although there are other targets that are being explored in clinical trial.
Then we need to put these back into a patient. But before we do, we have to create what we call immunologic space, because people have a repertoire. They have all different types of T cells. And if you just infuse the new attacking ones in there, there’ll be a voice in the crowd, a drop in the ocean.
CAR T cell therapy process
Dr. Joshua Richter: So first we give something called lymphodepleting chemotherapy. Low doses of two drugs, cyclophosphamide and fludarabine. That clears out the T cells. And then we infuse the engineered T cells to attack the cancer.
This is a very effective and very powerful new tool. At the moment, we have one approved. It goes by the name of ide-cel, or idecabtagene vicleucel,ABECMA, and giving this drug or this therapy requires a two week admission into the hospital.
Ide-cel’s side effects and how to manage them
Dr. Joshua Richter: The major toxicity is something we call CRS or cytokine release syndrome. So when we infuse these T cells, they send out and they connect and communicate with other cells in the body through chemicals we call cytokines. And these cytokines activate the cells. And sometimes you can get an overactive immune system.
We have different techniques now to kind of quell to get kind of like the Goldilocks version. You don’t want the porridge to be too cold. You don’t want it to be too hot. You want just the right amount of immune activation where it kills the cancer cells but doesn’t get too active. So we try to walk that line.
And it’s interesting. Many of the ways we do that are similar approaches to how we manage COVID, because COVID can cause an overactive immune system. So there’s a lot of parallels there.
This was commercially approved in March 26th of this year. Another one is expected to be approved in November. And for patients who’ve had multiple lines of therapy, this type of technology is one of the best things that we can offer. And we’re just extremely excited about it.
The Patient Story: That was that was well described, Dr. Richter, I’d love to see if Dr. Gasparetto has anything to say with your experience.
Dr. Cristina Gasparetto: I think it’s a new way to treat myeloma. And one of the advantages of the current T cell is, it’s a one time deal. The last patient I treated, she unfortunately didn’t remain in remission for a long, long time. During that time, she was really active. She was happy that she was not receiving any additional therapy. And so that is definitely a plus.
Research on how to prolong response
Dr. Cristina Gasparetto: The problem is that unfortunately, because it’s a one-time deal patients eventually they lose the capability, the immune system. So now there is a lot of research involved on maybe designing these CAR T a little bit differently so there is more sustainability.
Maybe we need to do some maintenance. Maybe we can use it a couple of times. It will, of course, like all the other drugs that are entering the field of myeloma, initially, they are tested on very heavily pretreated patients.
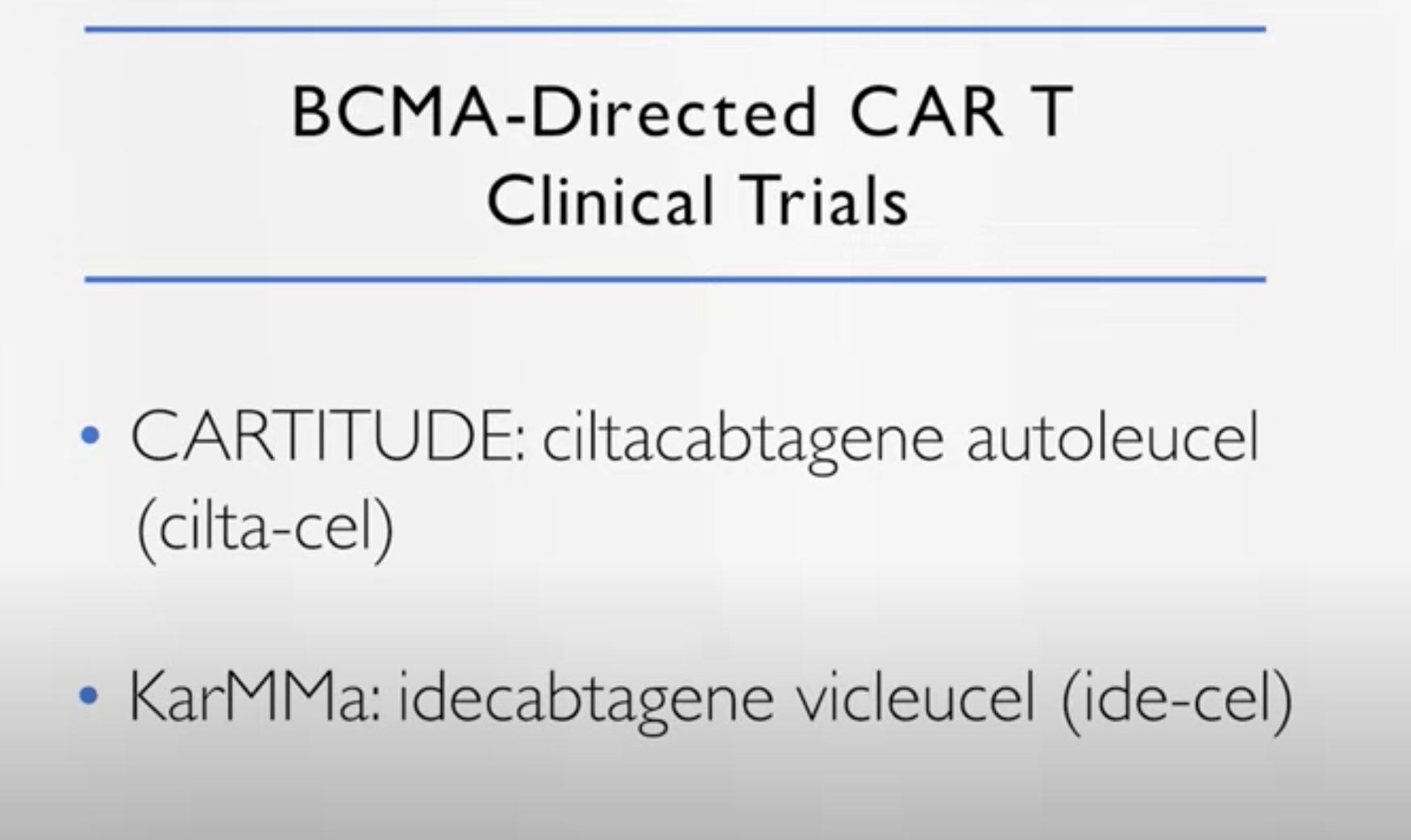
So they are, of course, the first one to be approved. If you look at old patients the durability of response, they were a little shy of a year but quite impressive responses. So if we can move these approaches earlier and we saw that with the cilta-cel, with the CARTITUDE.
The CARTITUDE has a little bit different construction when it was used to treat the patients, the durability was maybe a little bit better, but similar to the KarMMa.
Treating patients earlier with CAR T
Dr. Cristina Gasparetto: But now in patients treated earlier, we see amazing responses and the durability is getting longer and longer, particularly with patients achieving complete remission, the MRD that we were talking about earlier.
Can we use the MRD in this situation? And in addition, the CRS, we started to also get the feel of what we called neurotoxicity. There are different types of neurotoxicity. The earlier on that we can treat with steroids, tocilizumab, et cetera.
The overall response to ide-cel so far
I think we are making these CAR T cell safer and safer. I was impressed by the overall response, the MRD and the fact that the toxicity profile is becoming, you know, we’re testing now some of these CAR T cells exclusively in the outpatient setting.
Before when we started, Joshua said patients had to stay in the hospital to be monitored for these major side effects. But now we can manage them in the outpatient setting. So they’re becoming safer.
We’re moving them earlier. Maybe some maintenance may be a boost in a way. And so it’s a new way to treat myeloma. We’ll see, over the next few years, if we can use them up front for those at risk. If we can give them after transplant, etc. But very, very excited having these two major procedures available for all patients.
When should multiple myeloma patients and their doctors consider CAR T
The Patient Story: Thank you, Dr Gasparetto. You know, Dr Richter, as Dr. Gasparetto was saying, when you get CAR T will be used hopefully earlier in certain situations. But right now, I think there were some questions from the audience about how do I know when to talk to my doctor, especially if they’re not at a center like yours,about whether I should pursue one of these combinations, you know, and go in that direction versus when is CAR T right for me? So on a high level, how would you describe that?
Dr. Joshua Richter: The earlier, the better. And the reason why, the earlier the better is that right now there is one commercially available CAR T, but we still have many CAR T’s in clinical trials and slots in those trials come and they go. So being on someone’s list at an institution that does them is a really great thing.
The other issue is it’s worthwhile to have this communication between your local physician and the myeloma CAR T physician, because that’s right now CAR T’s are approved for four or more lines of therapy. But we’re starting to learn that the therapies you get leading up to that may affect the ability to collect and engineer those T cells.
For example, melphalan, which is a drug we absolutely love, if you get a good slug of melphalan prior to when we would need to collect T cells, we may not be able to collect enough to engineer.
So, you know, kind of making sure that is exactly as Cristina talked about earlier, that sequence that we’re all trying to figure out for each individual patient, trying to follow some relative do’s and don’ts so that if and when it’s time for a CAR T, you’re in the optimal position to do it.
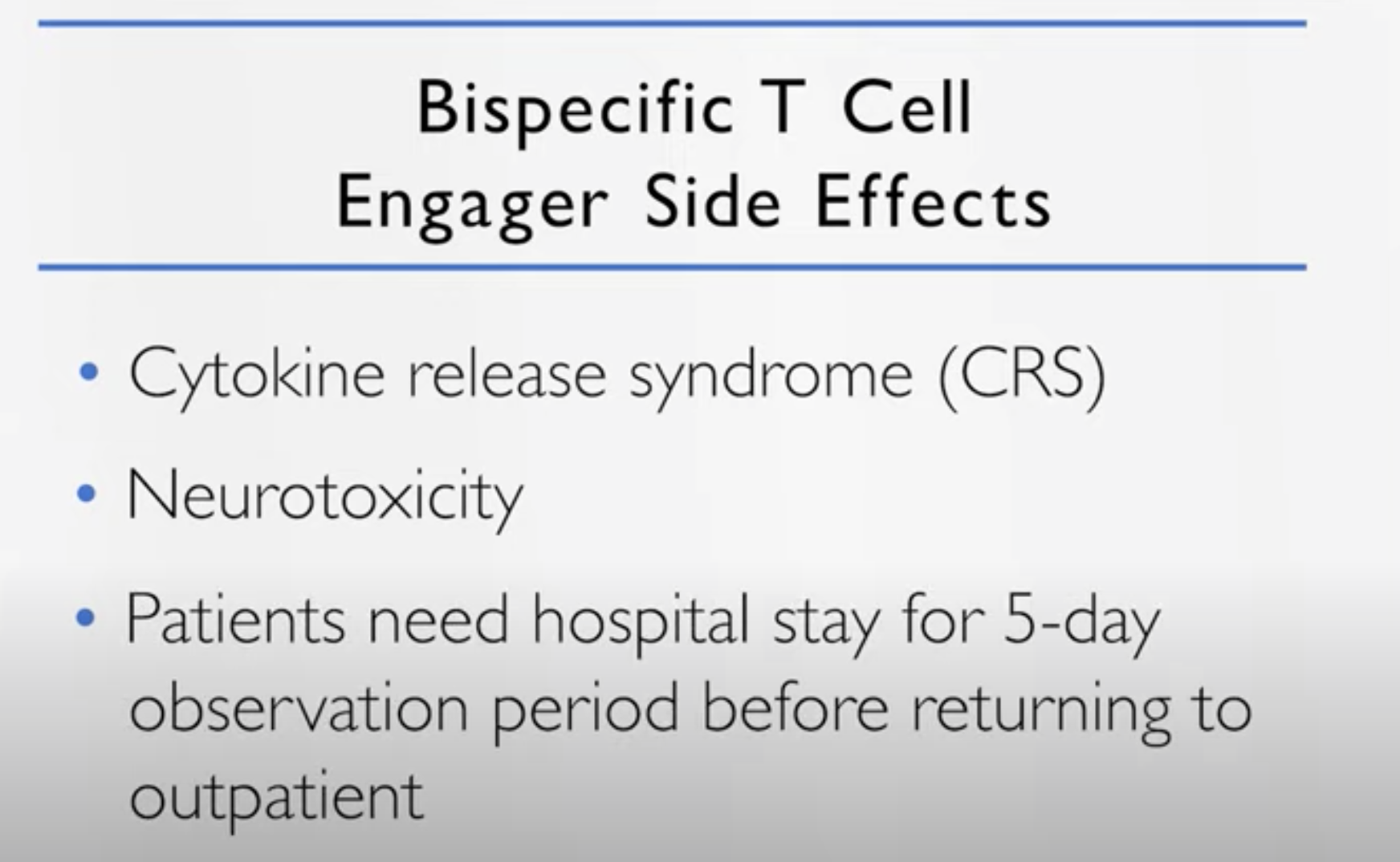
Bispecific T Cell Engagers (bispecifics)
The Patient Story: So I’d love to now go into an area a lot of people have been sort of hearing about, don’t know as much about, which is the bispecifics. So if we could just set it up again, in layman’s terms, what those are, what it means and maybe how patients can view them as a treatment option. And we’ll start with you, Dr. Gasparetto.
What are bispecifics and how do they work?
Dr. Cristina Gasparetto: These are the new antibodies. So, of course, like we were talking about the dara, the isatuximab, the belantamab, so they have different targets. And the reason why they are a little bit different, because in addition to the target, the myeloma and one of the most used targets is the BCMA. But we have different targets, actually different bispecific antibodies, different targets.
But the other one,they engage the T cells. So sometimes I explain to patients it’s maybe a way to do CAR T cell in vivo, in the person. So you don’t engineer, you don’t take the lymphocytes out but you’re trying to stimulate the lymphocyte in. So it is very important for this type of therapy to have a relatively good immune system.
We’ll see these therapies going a little bit earlier now and be tested. But we are all surprised, we’re all kind of excited by the preliminary results because I was involved with the elranatamab, I can’t pronounce it.
The Patient Story: Can I just say it’s very refreshing to hear that you can’t pronounce some of these things!
Dr. Cristina Gasparetto: No, I know, because we have all these names – elranatamab. I have to think about this. And it’s actually initially we are using these drugs. You know, again, engaging the T cells, attacking the myeloma. So that is the effect of the immune system directly.
Best administration for bispecifics
Dr. Cristina Gasparetto: They have other mechanisms of action, of course. And so what we’re learning is that we can give them IV in the vein, but the subcutaneous is becoming actually very for all these drugs, probably the better route of administration.
Bispecifics common side effects
Dr. Cristina Gasparetto: We are trying to find the right dosage. Also the toxicity of these drugs is very similar to the CAR T cells. So we see the cytokine release syndrome, we see the neurotoxicity. And so at the beginning, some patients in some trials are admitted to the hospital for observation for a few days, five days, and then they could continue the therapy in the outpatient setting.
So the difference with the CAR T cell, the CAR T cell is one time and then and you’re engineering your lymphocytes. With these bispecifics you have the continuation.
So which one is going to be better? The continuation versus or the convenience of doing one time.
Elranatamab clinical trial response
Dr. Cristina Gasparetto: We see tremendous responses with the elranatamab. We had responses in about 70 percent of patients with one-third, 30 percent achieving a complete remission. Now, we don’t have long term follow up to see if these are sustainable.
We don’t know yet but we are very impressed by the early results. And so there are a lot of new bispecifics. They are being designed. Different targets engaging the T cells, it’s part of the immune system and definitely is going to change how we treat myeloma, in my opinion, over the next few years.
So very interesting results. Very similar toxicities with the CAR T cell, great responses. But we don’t know enough about durability. What do you think, Joshua? It is premature, but we are all excited.
Dr. Joshua Richter: I couldn’t agree more. I mean, to me, I think your statement of we don’t know which one is the optimal one for an individual patient is perfect. The one advantage that bispecifics have is a theoretical possibility of being given in the community.
The bulk of myeloma patients are treated in community setting
Dr. Joshua Richter:
In the United States, 70 to 80 percent of myeloma is treated in the community.
So despite the fact that Cristina earlier today probably had a quadruple booked clinic for every single time slot, within the United States, most people get treatment locally.
CAR T’s are probably always going to be reserved for the big centers, but as we get better at managing the CRS with these bispecifics, there may be a point in time where they can be given in the local community practice, which would be just available to more patients, which is an amazing thing.
Different bispecifics with different targets
Dr. Joshua Richter: The other thing is the different targets we have. So all bispecifics, they have two arms. One is CD3, which grabs onto the T cell and the arm that grabs onto the cancer cell. Right now in our clinic, we have four different targets. That’s the other part of it.
One is BCMA, which is the same target as the CAR T cell and as belantamab. One is CD38, So that same target as isatuximab or daratumumab.
Talquetamab and cevostamab
Dr. Joshua Richter: There’s a drug called talquetamab. Talquetamab has this arm here that targets something called GPRC5D, which is on all myeloma cells.
And then there’s another drug called cevostamab with this arm that has something called FcRH5 which is also on all myeloma cells.
And these different markers are independently expressed on the cancer cells. So there is a potential there to sequence these different drugs. We have no idea yet. Does BCMA go before CD38 or after GPRC5D? We don’t know any of that.
But the great thing is as we get these drugs approved, we will all, as a community, learn what the optimal strategies are.
Dr. Cristina Gasparetto: And there is also one that we’re participating with the CD38, so like the daratumumab target. And actually with the elranatamab we had about 20 percent of patients previously exposed to other BCMA targeted therapies, and we still had the response there.
So I think that another big question for us is:
- Can I use belantamab?
- Can I use the bispecific?
- Can I use the CAR T cell with the same target?
- So what are accumulating data information about?
- Can we sequence these drugs and still maintain efficacy?
Because right now it’s a little bit all over the place. We don’t know.
The Patient Story: So much data coming out constantly. So what I’m hearing from you both is, you know, with bispecifics. Really importantly, Dr. Richter, the fact that I didn’t know it was 70 to 80 percent specifically for myeloma patients being treated in a community setting. That’s incredible. So that would help with that population, which would be huge.
There’s also the issue of sometimes with CAR T. Is it true that you have to bridge. Well, yeah, with another treatment. I mean, there’s variations as you’re waiting for the reengineering to happen and then infusing it back into the person. I mean, I guess maybe a summary of the pros and cons of both would be great. And we’ll start with Dr Richter.
Pros & Cons: CAR T vs. Bispecifics
Dr. Joshua Richter: The pros of CAR T’s is that it’s a one and done. But as you said, right now, all the CAR T’s that are either approved or about to be approved are what are called autologous. We have to have manufacturing of someone’s individual cells, which can take upwards of five weeks for some people. There are ongoing studies looking at allogeneic CAR T’s, which would be an off the shelf option, which would negate the need for bridging.
The pros of a bispecific is they, at some level, can be given in the community, which may make them more advantageous. And because it’s maybe a little more titratable in terms of the side effect profile. You could probably give a bispecific to a little bit of an older or frailer patient, but we don’t have all that information just yet. I don’t know if Cristina has any better way of putting it.
Dr. Cristina Gasparetto: I think that for now, the CAR T cell is limited to the larger academia large institutions. And you’re right that we may not be able to offer the CAR T cell to the frail, immunocompromised, very difficult patient to treat.
So the bispecific, out of the shelf. But you know, they have to sustain therapy, and most of them are given weekly or every other week or so. But maybe that will translate to longer durability of responses that, you know, we don’t know. So right now, we are still accumulating data. Can we use all of them?
You know, eventually our only type of combinations or so. But the good news is that we do have a lot of options available and that, when we are called to talk about the relapsed refractory myeloma, I don’t know if you have tried to make a slide deck recently, but is impossible because we have so much data, so many options.
And it’s impossible. I don’t want to talk about the relapsed refractory because it’s crazy to keep up with everything, which is great, right? It’s easy to talk about. No, I don’t know. Transplant, a one time shot. But there are lots of options. And, you know, we say a lot. But I think having a referral to a myeloma specialist, I agree with Joshua, is so important because we are aware of what’s going on, even behind the scenes.
And at least we know. And if we don’t have a particular trial available in our institution, you know, we know who does it, and we can refer patients to each other and continue the treatment.
What does it mean to be offered clinical trial participation?
The Patient Story: Perfect. And we’re going to end with an audience question. And you can just both respond and then that will be it for the day. And we’ll hopefully get some follow up questions later. But the question is from Richard D. His wife has been through a few lines of treatment. Another oncologist has brought up a new clinical trial.
I don’t have the details, but the question is, do doctors only bring up a clinical trial when the situation is hopeless? My wife is feeling upset and nervous about what this means for her/us.
I think what this brings us back to you is that there’s all this data and it’s great having these conversations. But when people hear certain phrases, sometimes it can be really overwhelming and very daunting, like what does this mean for me? So with that, maybe we’ll start with you, Dr. Gasparetto. What would you say to someone?
Dr. Cristina Gasparetto: I would say just keep it in perspective. I mean, so if they’re offering a clinical trial, it’s because the patient is still eligible for clinical trials. So that is good because we have stringent eligibility criteria, because the patient has to be in good shape, having good counts, good renal function so we can test a drug.
But I think about all these drugs that were just presented. They were all in clinical trials. And we have many success stories. We have patients in remission. A patient responded phenomenally.
I am pro clinical trial because it’s the only way that we can learn how to treat myeloma and other cancers. And so no it’s not a bad thing. It means that the patient is eligible for something that may be even more powerful.
We don’t know if we don’t test. We test a very powerful combination. So even if you are in a clinical trial comparing one old versus one new combination, they are very effective. We don’t design a clinical trial to have one of the combinations completely failing.
So I think that, you know, keep things in perspective. If the patient is eligible for a clinical trial, it may be a good thing. I mean, you know, a good marrow, sort of a good renal function and maybe new drugs, who knows? A new combination that can be really important for a cure for the patient and for other patients in the future.
Dr. Joshua Richter: I completely agree with Cristina. I am very pro clinical trial. And in fact, the earlier the better exactly as Cristina talked about. If you go through every treatment available, you may then no longer be eligible for any clinical trial.
The benefit of doing a clinical trial early on is that if it works, you get access to a standard of care. Five years from now, you get access to it now.
And the other benefit is that if it does not work, you have all of the currently approved drugs ready to go at a moment’s notice that you can salvage with if you need. And every moment you are on that clinical trial, the bucket you can reach into for new therapies grows and grows.
If she goes on that clinical trial today and is still in remission until December, we will have cilta-cel approved before then. If she stays in remission next year, we’re going to have CELMoDs approved and bispecifics improved. And that means that standard of care option bucket just grows larger and larger.
Cristina hit the nail on the head. Pro clinical trial involvement. We are all about it. It deepens our knowledge about how to treat an individual, how to treat everyone as a whole. And the simple fact is, the myeloma world is one big community.
If there is a trial, I do not have it, I know who it is and will refer them out. We have patients that move. So I had a patient that recently moved to Florida. I called up our colleagues at Sylvester Cancer Center in Miami. They’re going to see them. So taking advantage of the myeloma community that we all have for clinical trials is absolutely the right way to go.
The Patient Story: That is so well said. Thank you so much. And I want to thank you both for spending so much time with us today. Not only doing the work that you do in clinic and in research, but for things like this where you are reaching people who are not your own patients. And so very, very grateful for that. Doctor Cristina Gasparetto, Dr. Joshua Richter, thank you so much for joining us today.
Dr. Cristina Gasparetto: Thank you. Thank you for having us. And we love to treat patients that, you know, it’s a love hate affair, like I mentioned. So of course, this kind of discussion and conversation are always the best even for us.
Dr. Joshua Richter: Absolutely, it’s always a pleasure to work with community advocacy groups and to get to hear from the great Cristina Gasparetto.
Dr. Cristina Gasparetto: You, too. When, of course, when she told me that I was working with you, I was very happy.
Dr. Joshua Richter: So same here.
The Patient Story: Well, it’s been a great conversation. And for everyone who wants to watch this discussion again, just go to ThePatientStory.com where we give human answers to your cancer questions.
Other Multiple Myeloma Experts
No shortCode found